


INTRODUCTION
Renal cell carcinoma (RCC) accounts for 4.2% of adult malignancies and is the 10th leading cause of cancer deaths in the United States.1 In the United States in 2024, more than 81,000 new cases were diagnosed and more than 14,300 died, due mainly to metastatic disease.1 (See SEER: https://seer.cancer.gov/statfacts/html/kidrp.html). Globally, there are more than 400,000 cases of kidney cancer,2 and incidence of kidney and renal pelvis cancers increased between 2010 and 2019,3 with a dramatic increase among younger individuals attributed to obesity.4,5
Kidney cancer is a stealth disease because it is typically asymptomatic. When diagnosed symptomatically, it is detected extremely late in its clinical course and has already metastasized in 30–40% of patients.2 Kidney cancer is resistant to chemotherapy, and metastatic disease portends a miserable prognosis. The 2-year survival is only 18%,6 and 5-year survival is 5% or less.1,2 Even with immunotherapy, survival is low and variable.7
Early kidney cancer detection enabling prompt intervention, ideally before symptoms and metastasis, enables the highest likelihood of preventing death. Unfortunately, there is currently no cost-effective method for screening asymptomatic populations. With the ubiquity of diagnostic abdominal imaging (computer-assisted tomography, ultrasound, magnetic resonance), about 80% of kidney tumors are now discovered incidentally.2,4 Despite this, even with earlier detection, up to 30% of patients with no discernible metastasis at nephrectomy will eventually have metastatic disease.2,4 Conversely, there is a high fraction of normal kidneys removed at surgery for an imaged renal mass presumed to be cancer.
There is an unmet need for identification of effective biomarkers to reliably detect preclinical kidney cancer, differentiate cancer from benign renal masses, differentiate indolent from aggressive tumors, and identify or predict metastasis early enough to facilitate appropriate intervention. In an initial proof-of-concept study,8 we showed that urine concentrations of the angiogenesis-associated water channel protein aquaporin-1 and the lipid droplet-associated protein perilipin 2 in patients with clear cell and papillary RCC are significantly greater than controls, and both proteins significantly decreased upon tumor removal.9–13 Together, clear cell and papillary cancers account for about 80% of all kidney cancers. Furthermore, these biomarkers were not confounded by co-morbid non-cancerous kidney diseases,10 or by bladder or prostate cancers.12 These urine biomarkers mirror primary tumor burden.8–10,12 A limitation, however, is that they do not reflect tumor grade or predict metastatic potential.
The acute phase reactant C-reactive protein (CRP) was found immunohistochemically to be expressed in excised RCC tissue but not adjacent normal tissue.14 Increased tumor CRP was attributed to increased acute phase cytokine interleukin-6 production within or around the tumor.14–16 Patients with advanced stage and high grade tumors had plasma CRP concentrations that were greater than normal.14 However, there was no discernible CRP found in urine using the rather insensitive assay (µg/mL) available at that time.
In the present study, we measured urine CRP content of patients newly diagnosed with an imaged renal mass using a more sensitive (ng/mL) assay. We tested the specific hypothesis that, in RCC, urine CRP content is an early indicator of tumor grade and metastatic potential or subclinical metastatic spread. Thus, urine CRP can serve as a non-invasive liquid biopsy and may be used pre-surgically to grade tumors using molecular pathology. Additionally, we confined the study to patients with an imaged kidney mass of ≤4 cm, as these tumors are typically not thought to have metastatic potential.17
MATERIALS AND METHODS
Approval was obtained from the Washington University in St. Louis Institutional Review Board, and written informed consent was obtained from all patients. From July 2011 through December 2014, urine and citrated plasma samples were obtained from 65 patients preoperatively on the day of surgery who were undergoing partial nephrectomy based on a CT-imaged renal mass and a presumptive diagnosis of RCC. Post-operative pathology reports for those individuals provided cancer type, size, tumor stage (TNM classification, including node and distant metastases), and Fuhrman grade. Postoperative pathology diagnoses established clear cell RCC (55 patients) or papillary RCC (10 patients) with a tumor of ≤4 cm (T1a or T3a). Subsequently, 50 patients provided urine during their first post-surgical follow-up visit (3–6 weeks post-surgery). From July 2011 through December 2014, in addition to the 65 patients with RCC, pre-surgical urine was obtained from 7 patients post-operatively diagnosed with angiomyolipoma, 6 with chromophobe, and 9 with oncocytoma, all ≤4 cm. Urine and citrated plasma were obtained from 64 age- and sex-matched self-reported healthy volunteers.
From February through October of 2012, pre-operative urine was collected from 27 prostatectomy and 22 cystectomy patients with no RCC, which served as controls. Archived frozen urine from 25 prostatectomy (stage pT2c and pT3, Gleason grade 6–8) and 19 cystectomy patients (predominantly stage Ta, high grade, with no noted metastases) was used in this study.
Sample size calculations were based on urine CRP concentrations of healthy controls. Detecting urine CRP concentrations in RCC patients 2- or 3-fold (conservative assumption) greater than controls, based on a two-sided t-test and 80% power, would require a minimum of 40 (2-fold) or 10 patients (3-fold) at 0.05 significance.
Urine CRP concentration was measured by a sensitive and specific ELISA (DuoSet DY1701 kits, R&D Systems). The heightened sensitivity reduced the potential for confounding due to variations in urine pH or salt/urea excretion since urine was diluted 1:40–1:80 with assay buffer. Preliminary studies showed urine CRP dilution linearity paralleling the standard curve. Plasma CRP concentrations were determined after 1:1000–1:10,000 dilutions in assay buffer. Urine creatinine concentrations were measured as reported previously.8,11 Urine pH and specific gravity were determined using Siemens Multistix 10GS reagent strips.
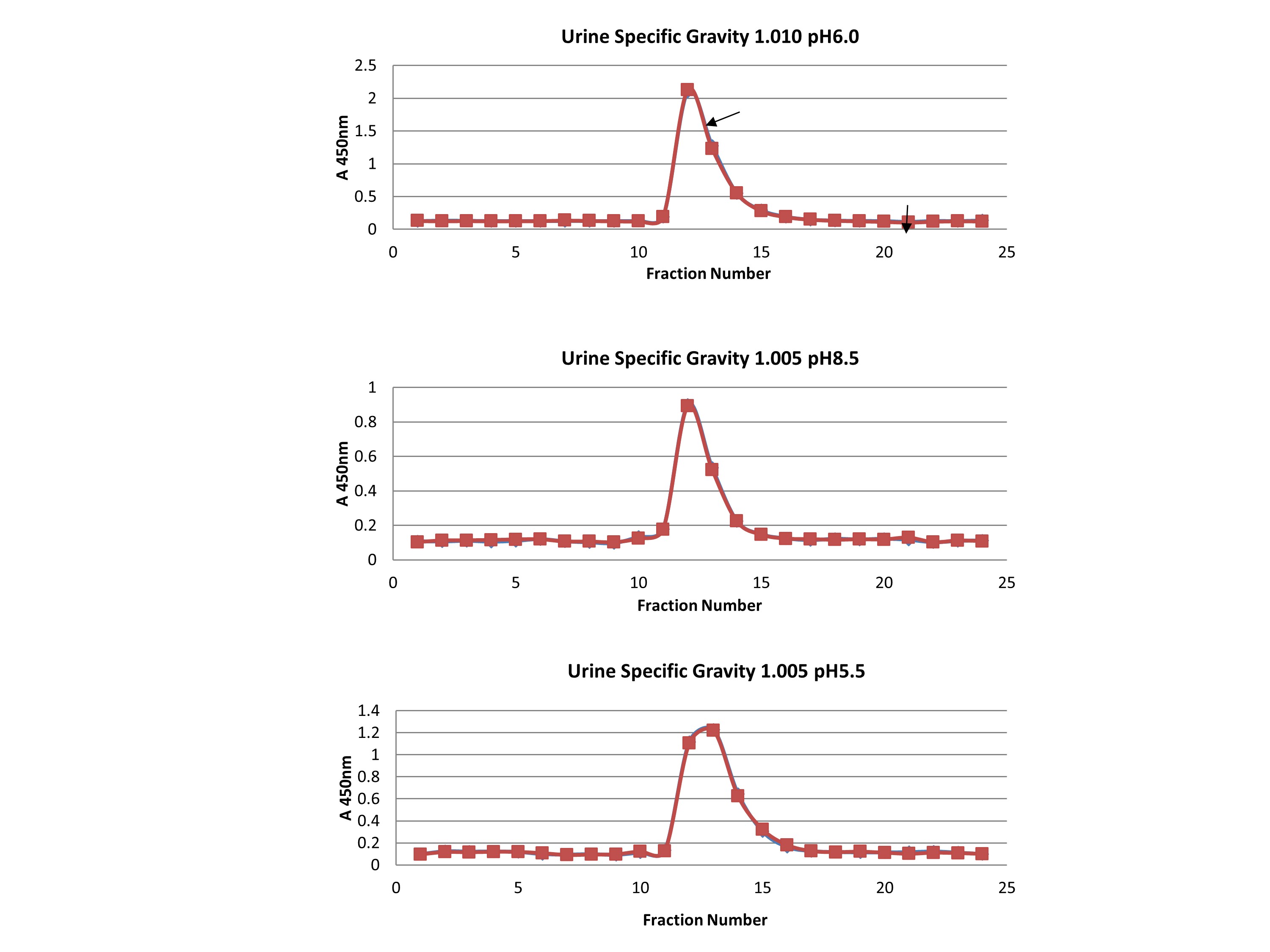
The molecular size of urine CRP detected by ELISA was measured to determine if it was the intact 125 kDa pentamer or 25 kDa monomers. Sizing was performed on a 0.7 × 30 cm Bio-Gel P-100 column equilibrated with sodium phosphate–sodium chloride buffers mimicking urine pH between 5.5 and 8.5 and salt concentrations mimicking urine specific gravities between 1.005 and 1.010 (Supplementary Figure 1). The column was calibrated using human IgG (150 kDa), human albumin (66 kDa), and human myoglobin (17 kDa).
Non-normally distributed variables were expressed as median with interquartile range and normally distributed variables as mean ± standard deviation. Age (one-way ANOVA) and sex (Pearson Chi-square test) were compared across groups. Urine CRP concentrations were compared using the Kruskal-Wallis test with Bonferroni correction. Receiver operating characteristic (ROC) analysis was performed using a nonparametric method. The cutoff of urine CRP compared to healthy controls was set at the lowest value of patients with grade 3 tumors to maximize sensitivity. Statistical analysis was performed using Analyse-it for Excel 2010 (Analyse-it Software, Ltd, Leeds, United Kingdom). Values were considered statistically significant at P<0.05.
RESULTS
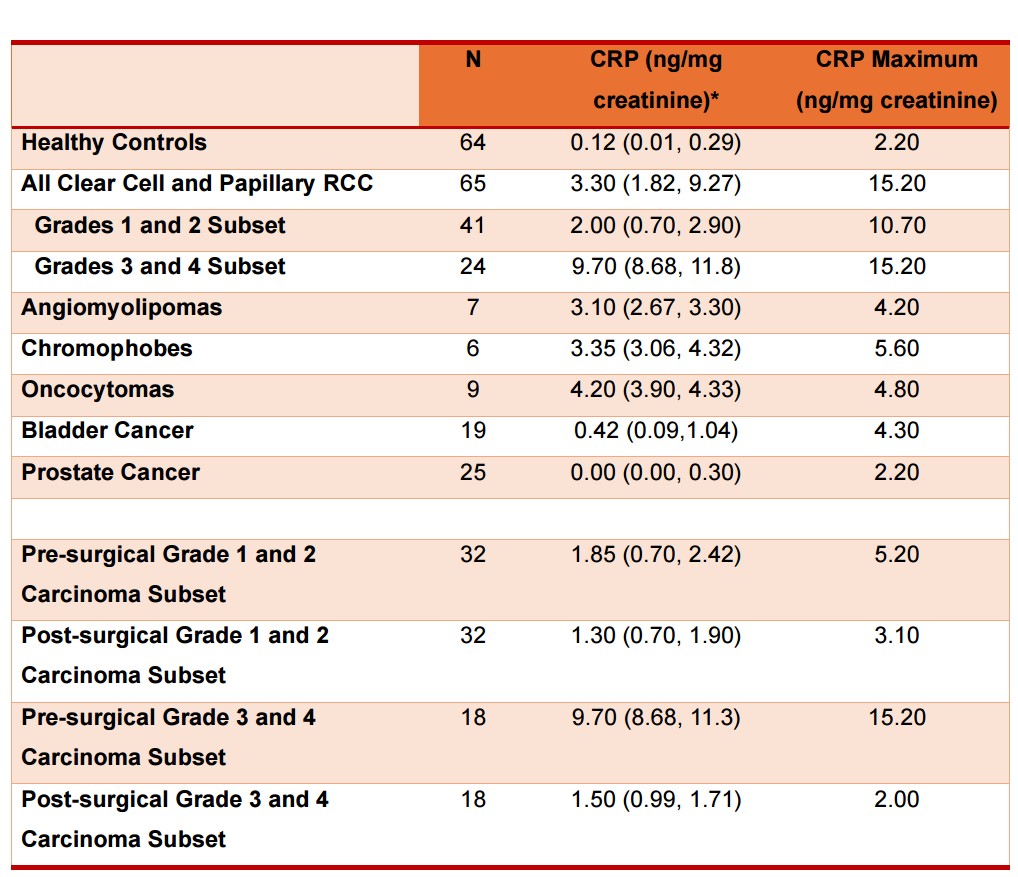
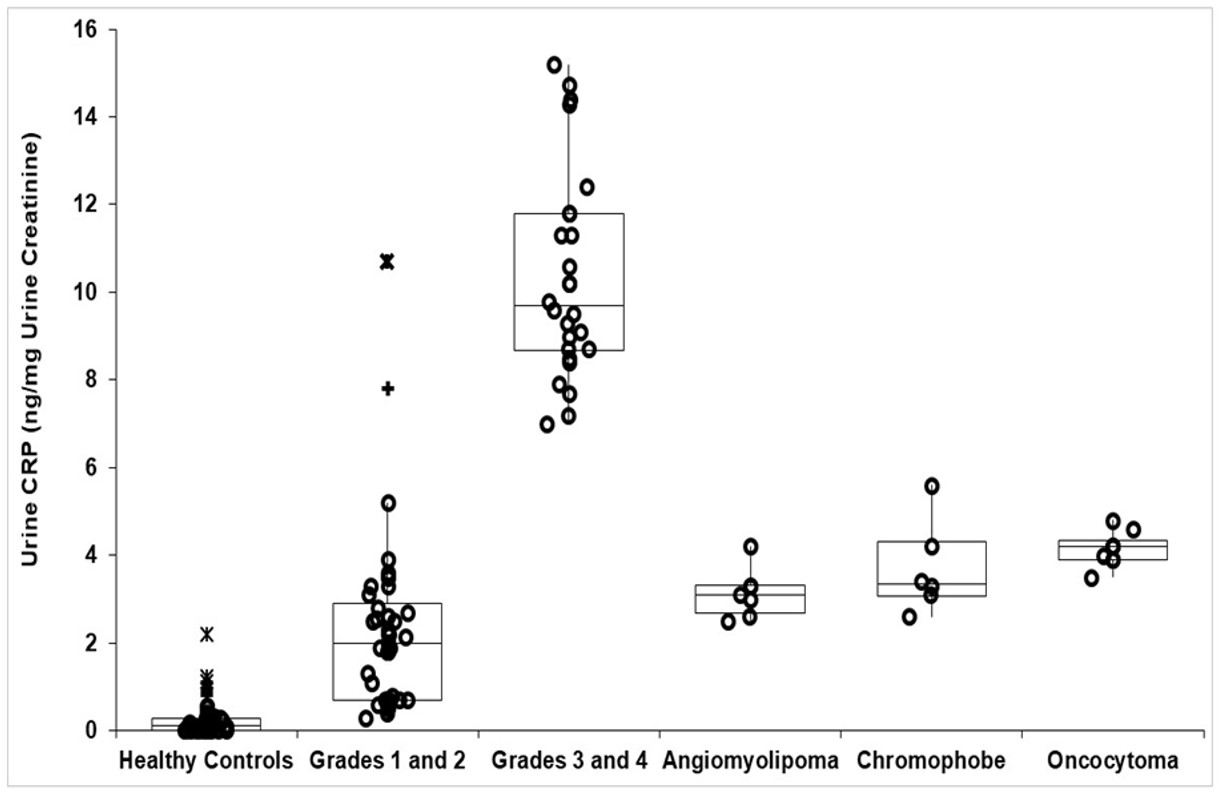
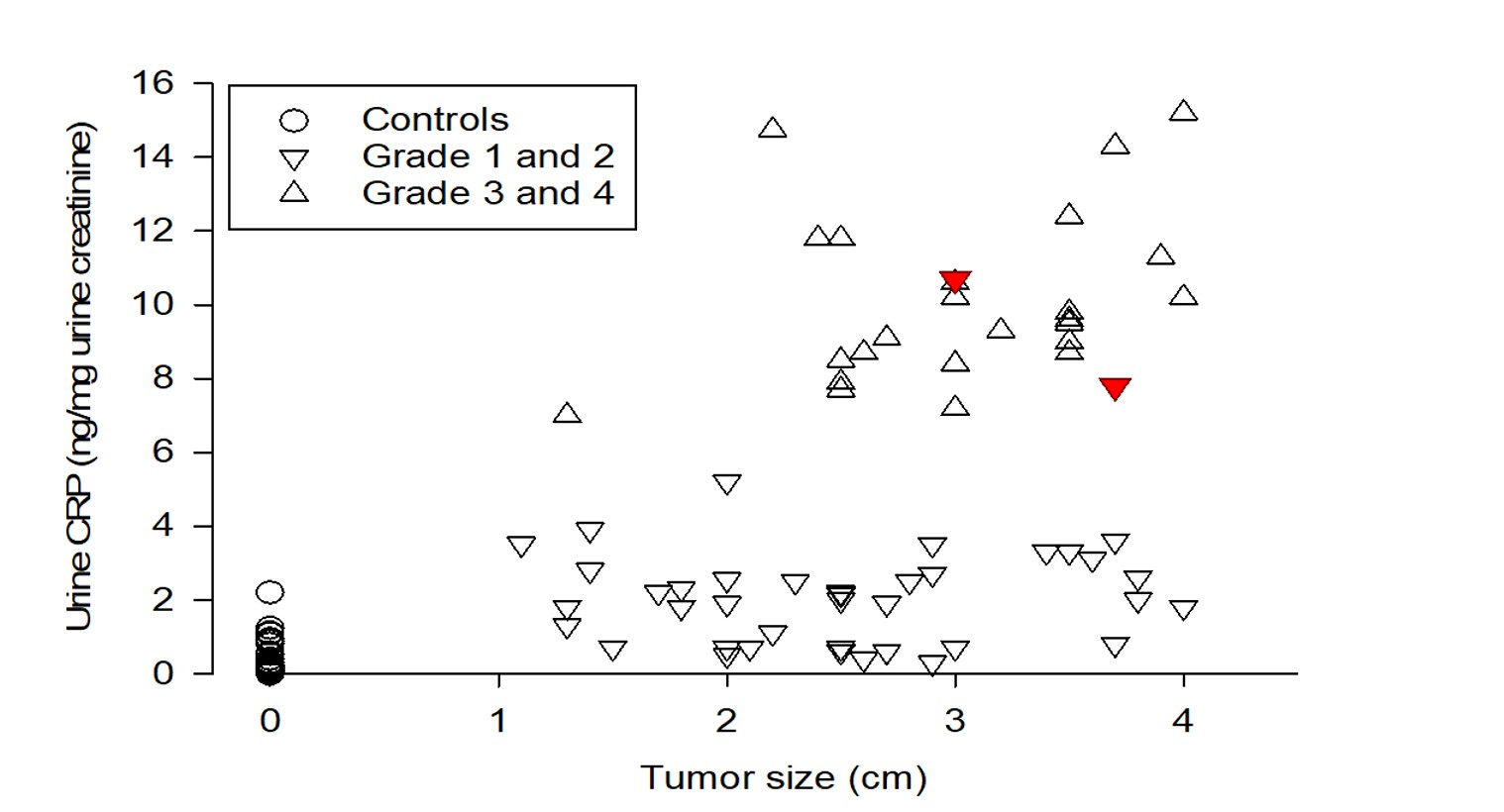
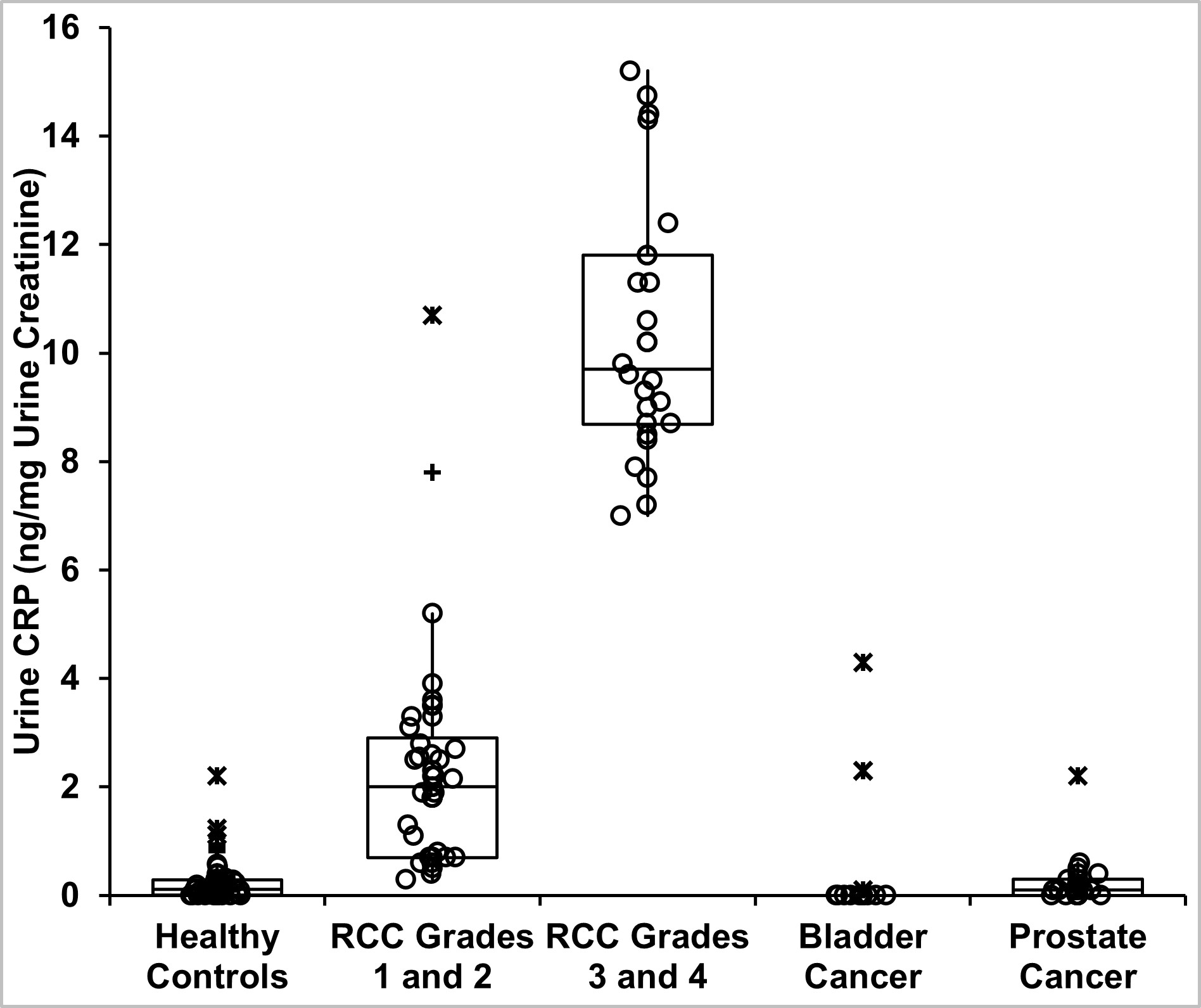
Table 1 provides demographics of patients and healthy controls. Urine CRP content in 65 patients with clear cell and papillary RCC and in 64 healthy controls is shown in Table 2. CRP in all RCC patients was 3.30 (1.82, 9.27) (median, IQR) ng/mg creatinine, which was 27-fold greater than healthy controls (0.12 (0.1, 0.29) ng/mg) (P<0.001, Mann-Whitney test). When factored by pathologic grade, the 41 patients with grades 1–2 tumors had CRP of 2.0 (0.70, 2.90) ng/mg, while the 24 with grades 3–4 tumors had 9.70 (8.68, 11.80) ng/mg (Figure 1, Table 2). These were 17- and 80-fold greater than controls (both P<0.001). Higher-grade tumors had ~5-fold greater urine CRP than lower grade tumors (P<0.001). Two outlier grade 2 patients had markedly higher CRP (Figure 1). Tumor size relationship is shown in Figure 2.
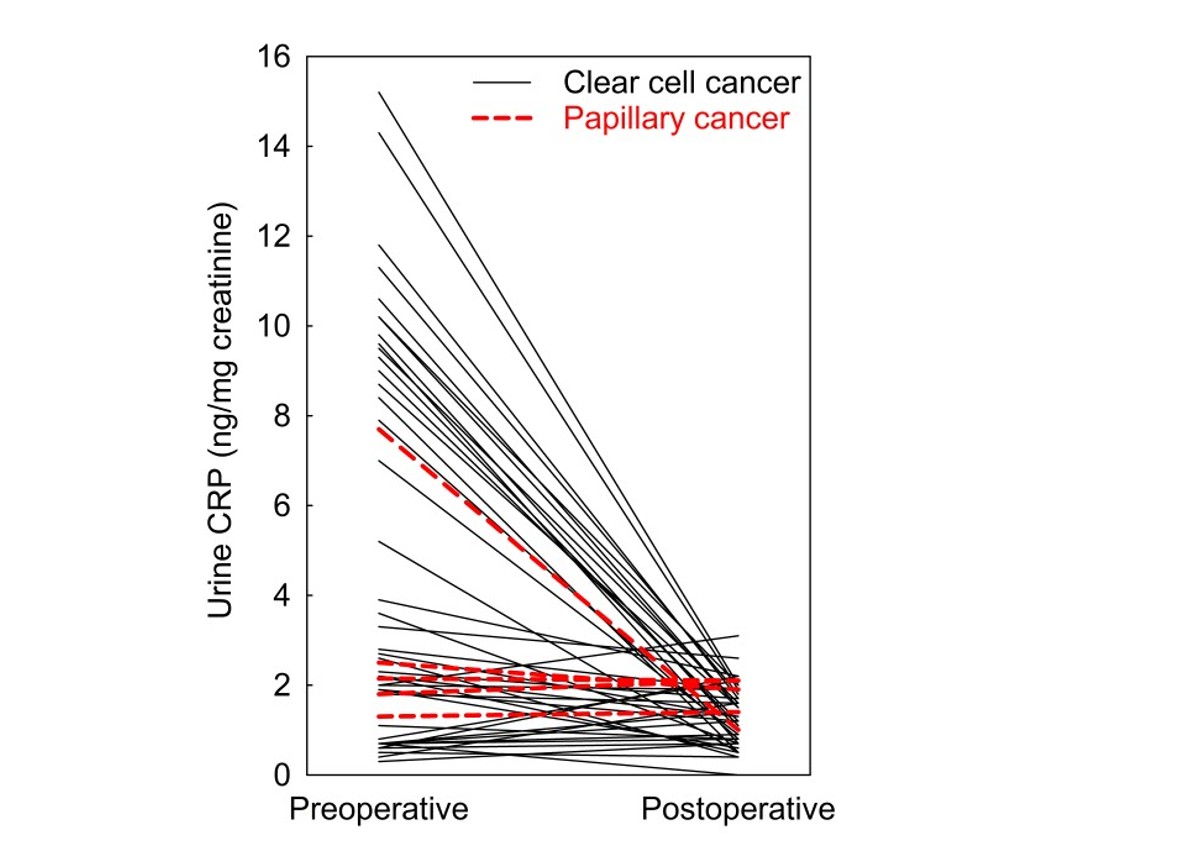
Biomarkers should reflect their tissue source and decrease after tumor removal. Of 65 RCC patients, 50 provided follow-up urine (32 grade 1–2; 18 grade 3). Urine CRP decreased 85% post-nephrectomy in grade 3 tumors (P<0.0001), consistent with tumor origin. No significant change occurred in low-grade tumors.
Molecular sizing showed urine CRP eluted at 125 kDa, with no monomeric (25 kDa) CRP (Supplementary Figure 1), indicating it is too large for glomerular filtration.
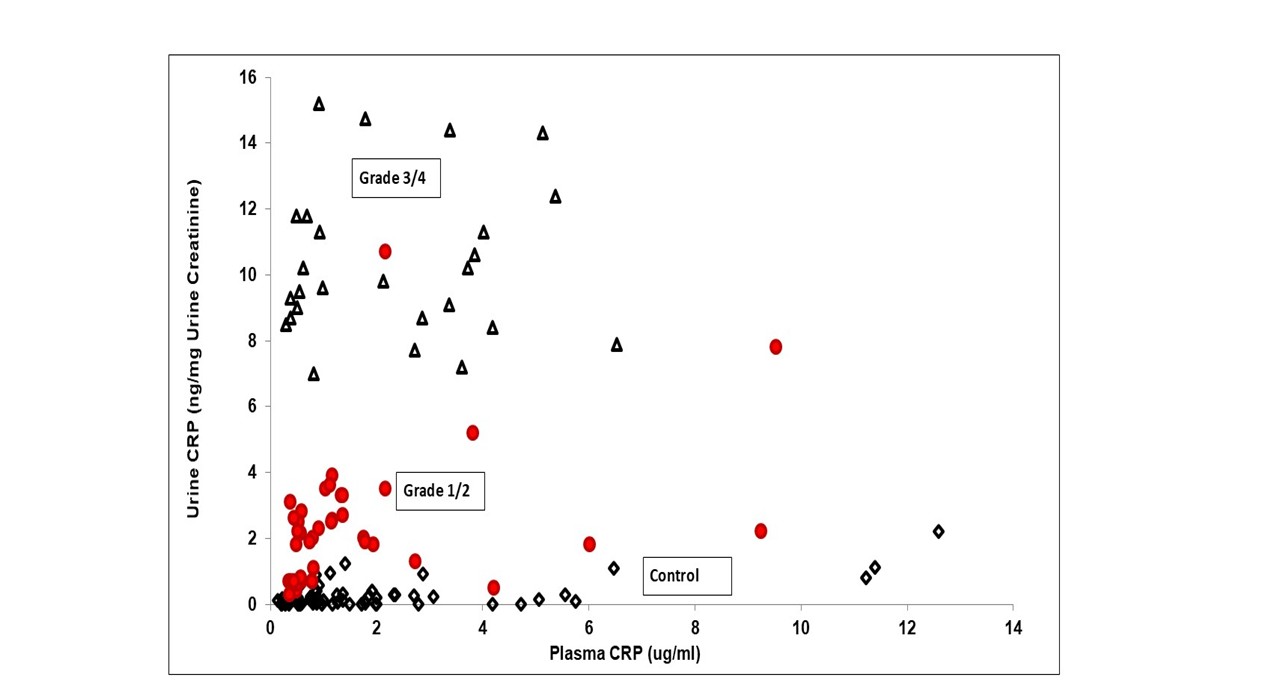
There was no correlation between urine and plasma CRP (Figure 4), suggesting urine CRP originates from tumor secretion rather than plasma filtration.
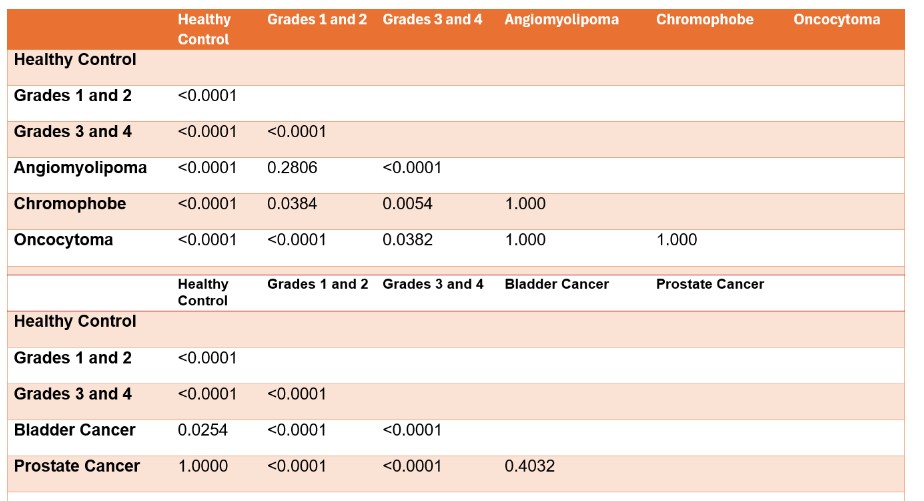
Urine CRP content in angiomyolipoma, chromophobe, and oncocytoma tumors was significantly lower than grade 3–4 RCC (Figure 1, Table 2). Urine CRP in bladder and prostate cancer was not elevated compared with controls (Supplementary Figure 2), supporting specificity for high-grade RCC.
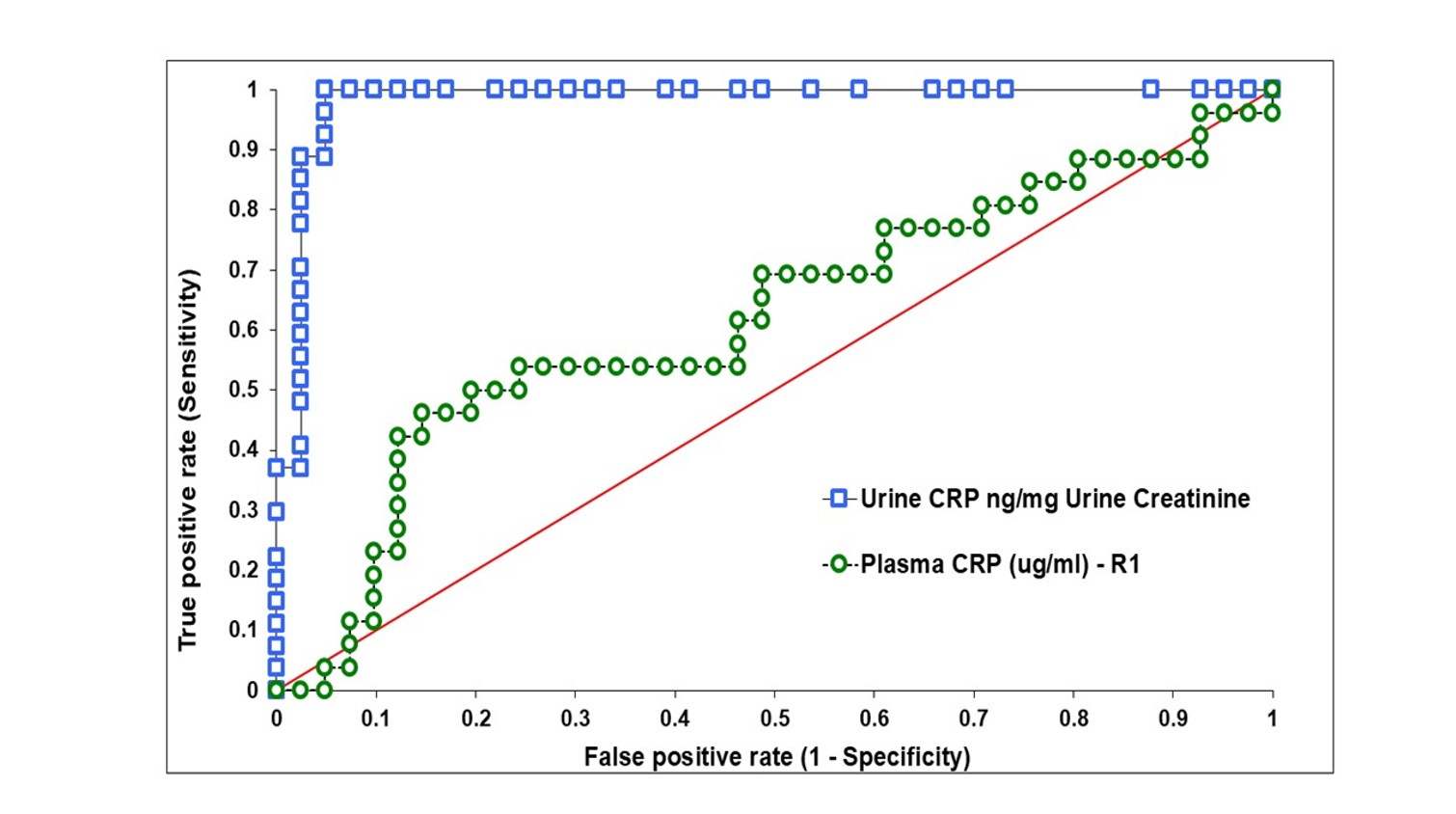
ROC analysis showed a cutoff of 6 ng/mg creatinine differentiated grade 1–2 from grade 3–4 RCC with AROC 0.98, sensitivity 1.00 and specificity 0.95 (Figure 5). Plasma CRP performed poorly (AROC 0.62).
Two grade 2 outlier patients with markedly high pre-nephrectomy urine CRP later developed metastases. All grade 3 patients available for follow-up developed metastases by 4.1 years (Figure 6).

DISCUSSION
This investigation tested the hypothesis that urine CRP is an early pre-surgical indicator of RCC tumor grade and metastatic potential, focusing on renal masses ≤4 cm. Urine CRP (but not plasma CRP) was a sensitive and specific measure of high-grade clear cell/papillary RCC and decreased after nephrectomy, indicating tumor origin.
Prior studies of plasma CRP and tumor CRP have had variable prognostic success. In small tumors, urine CRP provided superior discrimination of grade and potential metastasis. Elevated urine CRP likely reflects tumor IL-6 signaling and secretion into urine rather than filtration from blood. Complement factors may be less specific due to elevation in non-cancerous renal diseases.
Urine CRP may complement aquaporin-1 and perilipin-2 to provide non-invasive molecular grading of small renal masses and possibly identify occult metastatic risk. Larger prospective cohorts are needed to validate prognostic utility.
In summary, urine CRP was elevated in high-grade clear cell/papillary RCC and appeared specific versus other renal and urologic diseases. It may serve as a pre-surgical liquid biopsy for grading and metastatic risk prediction.
Additional Contributions
The authors thank Karen Frey, BS, RN, Jay McQuillan, BS, Jane Blood, BS, RN, Megan Kalin, MS, CCRP, Kalin Guebert, MS, CCRP, Michael Reeves, BA, for their expert help in the study. We thank Dr. Michael Abern for his critical review of the manuscript and insightful comments.
Conflict of Interest Disclosure
No author has any conflict of interest.
Funding/Support
NIH R01CA141521, Department of Defense CDMRP HT94252310996, Washington University in St. Louis Institute of Clinical and Translational Science UL1 TR000448, the Barnes-Jewish Hospital Frontier Fund (7537-55), Department of Anesthesiology, Washington University in St Louis School of Medicine, and the Bear Cub Fund of Washington University in St. Louis.
ETHICS STATEMENT
Approval was obtained from the Washington University in St. Louis Institutional Review Board, and written informed consent was obtained from all patients.
SUPPLEMENTAL INFORMATION
Please access the online content for full supplemental data: https://kidney-cancer-journal.com/KCJ23n3-or1.php
REFERENCES








