Editorial Perspective:
Shaping the Future of Adjuvant RCC Therapy
Practice-Ready Molecular Imaging in Renal Cell Carcinoma
Shawn Dason, MD & Eric A. Singer, MD
Division of Urologic Oncology, The Ohio State University Comprehensive Cancer Center, Columbus, OH
Corresponding Author
Shawn Dason, MD
7th Floor, 2121 Kenny Road
Columbus, OH, 43212
Shawn.Dason@osumc.edu
Abstract
Renal cell carcinoma (RCC) is frequently diagnosed incidentally, with conventional imaging modalities such as contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI). Although these modalities offer robust anatomical detail, they are limited in their ability to distinguish benign from malignant lesions. Molecular imaging represents an opportunity to characterize renal masses functionally. [89Zr]Zr-girentuximab PET–CT (girentuximab) targets carbonic anhydrase IX (CAIX), a marker specific to clear cell RCC (ccRCC). Girentuximab has demonstrated high diagnostic accuracy for ccRCC in the phase 3 ZIRCON trial, including a sensitivity of 85.5% and specificity of 87.0%. Clinical applications may include noninvasive diagnosis, surveillance risk stratification, and staging or surgical planning guidance. Separately, 99mTc-sestamibi SPECT/CT (sestamibi) differentiates oncocytomas from malignant tumors based on mitochondrial content, with pooled sensitivity and specificity of 89% and 91%, respectively. However, sestamibi has limited specificity for distinguishing oncocytoma from chromophobe RCC, which remains a clinical challenge. Despite this, sestamibi imaging offers value in reducing overtreatment of benign lesions and refining surveillance protocols. Both girentuximab and sestamibi are supported by growing evidence and are nearing clinical implementation. Future efforts should focus on integrating these molecular imaging strategies into standard care pathways.
Introduction
Renal masses are often detected incidentally through cross-sectional imaging, with contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) serving as the current clinical standards for diagnosis, staging, and surveillance (Campbell et al., 2021). While effective in assessing anatomy, these modalities offer limited capability to characterize tumor biology or distinguish benign from malignant lesions reliably. Molecular imaging, which leverages radiotracers targeting specific cellular pathways, represents a promising advance in renal cell carcinoma (RCC) diagnostics. Among the various investigational approaches, two agents—[89Zr]Zr-girentuximab (girentuximab) and 99mTc-sestamibi (sestamibi)—stand out as the closest to clinical applicability. Girentuximab, a monoclonal antibody targeting carbonic anhydrase IX (CAIX), enables specific imaging of clear cell RCC (ccRCC), while sestamibi has shown utility in differentiating benign oncocytomas from ccRCC based on mitochondrial content (Gorin et al., 2016; Shuch et al., 2024). Other molecular imaging strategies remain in earlier phases of development and are discussed in detail elsewhere (McKay et al., 2025; Wu et al., 2024).
Girentuximab
Imaging of carbonic anhydrase IX (CAIX) in ccRCC has been successfully achieved using radiolabeled monoclonal antibodies such as girentuximab, which specifically bind to CAIX expressed on tumor cells. CAIX is a hypoxia-inducible transmembrane enzyme that plays a critical role in pH regulation within the tumor microenvironment (Stillebroer et al., 2010). Its expression is nearly ubiquitous in ccRCC, driven by inactivation of the von Hippel–Lindau (VHL) tumor suppressor gene and subsequent stabilization of hypoxia-inducible factor-1α (HIF-1α) (Stillebroer et al., 2010). In contrast, CAIX is rarely expressed in normal kidney tissue or in non–clear cell RCC subtypes, making it a highly specific biomarker for ccRCC (Shuch et al., 2024).
The ZIRCON trial was a prospective, multicentre phase 3 study evaluating the diagnostic performance of girentuximab PET–CT imaging in detecting ccRCC among patients with indeterminate cT1 renal masses (≤7 cm) scheduled for nephrectomy (Shuch et al., 2024). A total of 300 patients received the tracer followed by abdominal PET–CT imaging, with histopathologic confirmation post-surgery serving as the reference standard (Shuch et al., 2024).
Among 284 evaluable patients, imaging demonstrated high diagnostic performance with a mean sensitivity of 85.5% (95% CI: 81.5–89.6) and specificity of 87.0% (95% CI: 81.0–93.1) (Shuch et al., 2024). Diagnostic accuracy remained high in smaller lesions, with sensitivity and specificity of 85.0% and 89.5% for tumors ≤4 cm, and 97.0% for both metrics in tumors ≤2 cm. Inter- and intrareader agreement were strong, with Fleiss’ κ of 91.1% and 100% intrareader concordance. No significant safety concerns were identified; most adverse events were unrelated to the tracer and occurred postoperatively. The most common tracer-related adverse events were fatigue (1.0%), nausea (1.0%), and abdominal pain (0.7%) (Shuch et al., 2024).
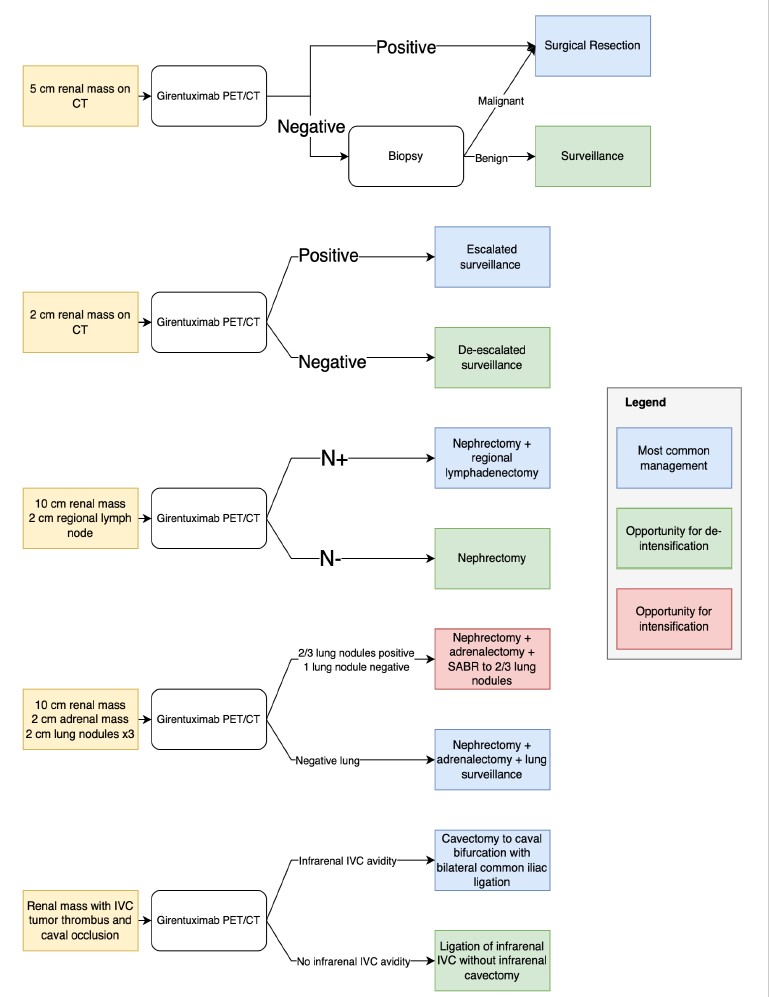
Girentuximab is under priority review at the United States Federal Drug Administration. If this agent becomes readily available, girentuximab PET–CT could become a routine part of diagnosing ccRCC. We await indication-specific data comparing girentuximab PET–CT to conventional imaging and biopsy, but could foresee its being used (Figure 1):
- Prior to the treatment of a localized renal mass. Around one-third of resected renal masses are benign (Kim et al., 2019). A similar proportion were historically ablated without pre-ablation confirmation of malignancy (Rivero et al., 2018). As a noninvasive option supporting a presumptive diagnosis of clear cell RCC prior to treatment, PET–CT may reduce overtreatment of benign renal masses.
- For risk stratification of lesions under active surveillance. Many renal masses under surveillance are too small or cystic for appropriate characterization with biopsy. PET–CT could assist in determining surveillance interval, modality, and decisions toward intervention.
- Decision to perform lymphadenectomy. While guidelines recommend lymphadenectomy at radical nephrectomy in the setting of lymph node abnormalities on preoperative CT, most are false positives (Campbell et al., 2021; Ray et al., 2023). PET/CT could assist in determining the extent of lymphadenectomy (Kaldany et al., 2020; Radadia et al., 2019).
- Staging in oligometastatic disease. Oligometastatic RCC management is nuanced (Dason et al., 2025). Patients suspected of oligometastatic RCC often have several equivocal abnormalities, and definitions of this state can be complex (Dason et al., 2023a).
- Assessing tumor thrombus. Distinguishing malignant versus bland IVC thrombus can be challenging and may guide anticoagulation decisions (Ray et al., 2023). Differentiating bland thrombus from retrograde-growing tumor thrombus is essential for tailoring resection extent (Dason et al., 2023b). The cranial extent of thrombus can also be difficult to delineate if not well shown by CT or MRI contrast.
Sestamibi
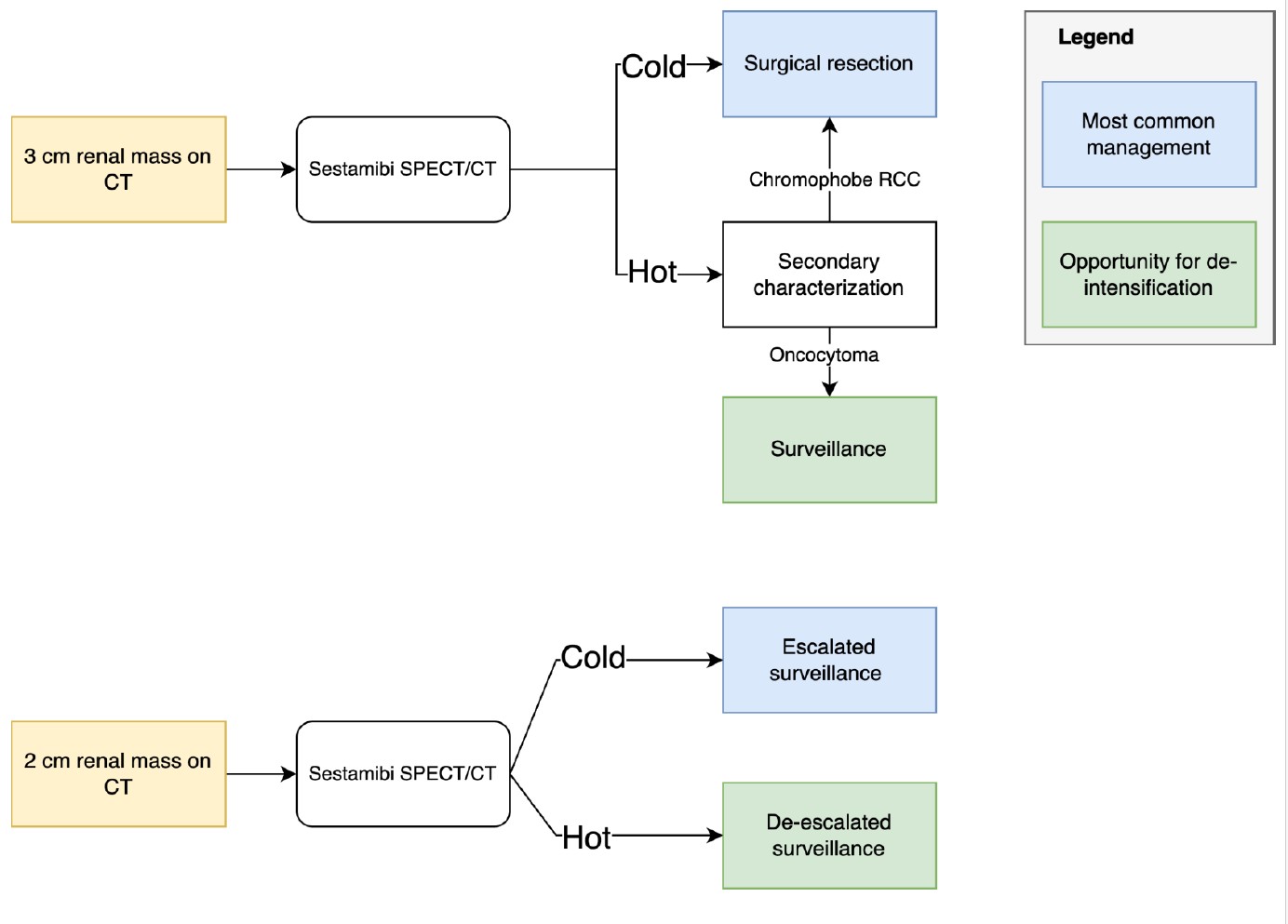
The radiotracer sestamibi preferentially accumulates in cells with high mitochondrial content, such as those found in benign renal tumors like oncocytomas and hybrid oncocytic/chromophobe tumors (HOCT), resulting in increased radiotracer uptake (“hot” lesions) (Gorin et al., 2016). In contrast, most malignant renal tumors, including clear cell and papillary RCC, exhibit lower sestamibi uptake due to reduced mitochondrial density and active efflux mechanisms, appearing as “cold” lesions. By fusing functional SPECT data with CT imaging, this modality allows for precise localization and characterization of renal lesions based on differential tracer retention (Gorin et al., 2016).
In a systematic review and meta-analysis by Basile et al., sestamibi SPECT/CT demonstrated high diagnostic accuracy in differentiating benign from malignant renal masses (Basile et al., 2024). Pooled sensitivity and specificity for identifying renal oncocytoma and HOCT versus all other renal lesions were both 89% (95% CI: 70–97% and 86–92%, respectively). When distinguishing oncocytoma/HOCT from clear cell and papillary RCC, specificity reached 98% (95% CI: 91–100%). However, specificity dropped to 41% (95% CI: 23–62%) when differentiating these tumors from chromophobe RCC. Overall pooled sensitivity and specificity for benign versus malignant lesions were 86% (95% CI: 67–93%) and 91% (95% CI: 87–95%), respectively (Basile et al., 2024).
A key limitation of positive sestamibi uptake is that it cannot reliably distinguish benign oncocytoma from malignant chromophobe RCC. Biopsy is also challenging for this distinction, as these entities may appear similar on core biopsy (Ng et al., 2014). Up to one-third of biopsies interpreted as oncocytoma are chromophobe RCC at resection (Patel et al., 2017).
Several radiologic options are being investigated to distinguish oncocytoma from chromophobe RCC. Using contrast-enhanced CT radiomics and machine learning, Li et al. achieved 94.5% diagnostic accuracy (AUC 0.964 ± 0.054) (Li et al., 2020). In a cohort of 76 patients, Patel et al. found that a CT PEER (peak early-phase enhancement ratio) cutoff of <0.60 differentiated chromophobe RCC from oncocytoma with 93.2% sensitivity, 87.5% specificity, and AUC 0.95 (Patel et al., 2022). In 18 renal tumors, Thorson et al. reported MRI-based PEER values ≤1.05 differentiated chromophobe RCC from oncocytoma with 100% sensitivity and specificity using averaged reader measurements (Thorson et al., 2025).
Notwithstanding these diagnostic challenges, separating oncocytoma from chromophobe RCC can sometimes be unnecessary clinically. Chromophobe RCC generally has a favorable prognosis, and small chromophobe tumors are often appropriate for active surveillance. Several series have noted no metastases during surveillance of oncocytic neoplasms (Baboudjian et al., 2022; Richard et al., 2016; Rodger et al., 2022).
Sestamibi could be used (Figure 2):
- Before treatment of a localized renal mass. A sestamibi-cold mass has a higher probability of malignancy. This characterization may reduce overtreatment of benign renal masses without the risks of percutaneous biopsy.
- For risk stratification under active surveillance. A sestamibi-hot mass may be surveilled less frequently than a cold one, potentially reducing patient anxiety and financial toxicity. A cold mass may prompt additional imaging and/or biopsy.
Given the rarity of advanced chromophobe RCC, sestamibi has a limited role in advanced RCC.
Conclusions
Molecular imaging represents a promising advance in diagnosing and managing RCC. [89Zr]Zr-girentuximab PET–CT has shown high accuracy in confirming clear cell RCC in suspicious renal masses. 99mTc-sestamibi SPECT/CT helps distinguish the most common benign renal tumor (oncocytoma) from the most common malignant tumors (clear cell and papillary RCC). With growing evidence supporting their utility, focus now shifts to integrating these tools into routine clinical practice. Urologists must remain good stewards of advanced imaging modalities and ensure equitable access to the potential benefits of molecular imaging.
References
- Baboudjian M, Moser D, Yanagisawa T, et al. Benefit and Harm of Active Surveillance for Biopsy-proven Renal Oncocytoma: A Systematic Review and Pooled Analysis. Eur Urol Open Sci. 2022;41:8–15.
- Basile G, Fallara G, Verri P, et al. The Role of 99mTc-Sestamibi SPECT/CT in the Diagnostic Pathway for Renal Masses: A Systematic Review and Meta-analysis. Eur Urol. 2024;85:63–71.
- Campbell SC, Clark PE, Chang SS, et al. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-Up: AUA Guideline: Part I. J Urol. 2021;206:199–208.
- Dason S, Lacuna K, Hannan R, Singer EA, Runcie K. State of the Art: Multidisciplinary Management of Oligometastatic Renal Cell Carcinoma. ASCO Educ Book. 2023;e390038.
- Dason S, Mohebali J, Blute ML, Salari K. Surgical Management of Renal Cell Carcinoma with Inferior Vena Cava Tumor Thrombus. Urol Clin North Am. 2023.
- Dason S, Wang S-J, Franceschelli D, Singer EA. Metastasis-directed therapy in oligometastatic and oligoprogressive renal cell carcinoma. Curr Opin Urol. 2025;35:194.
- Gorin MA, Rowe SP, Baras AS, et al. Prospective Evaluation of 99mTc-sestamibi SPECT/CT for the Diagnosis of Renal Oncocytomas and Hybrid Oncocytic/Chromophobe Tumors. Eur Urol. 2016;69:413–416.
- Kaldany A, Leopold ZR, Kim JE, et al. Dissecting the role of lymphadenectomy in renal cell carcinoma. Kidney Cancer J. 2020;18:103–108.
- Kim JH, Li S, Khandwala Y, et al. Association of Prevalence of Benign Pathologic Findings After Partial Nephrectomy With Preoperative Imaging Patterns. JAMA Surg. 2019;154:225–231.
- Li Y, Huang X, Xia Y, Long L. Value of radiomics in differential diagnosis of chromophobe RCC and renal oncocytoma. Abdom Radiol. 2020;45:3193–3201.
- McKay RR, Eapen R, Wulff-Burchfield EM, Hofman MS. Emerging Paradigms in GU Cancers. ASCO Educ Book. 2025;45:e473862.
- Ng KL, Rajandram R, Morais C, et al. Differentiation of oncocytoma from chromophobe RCC. J Clin Pathol. 2014;67:97–104.
- Patel HD, Druskin SC, Rowe SP, et al. Surgical histopathology for suspected oncocytoma on biopsy. BJU Int. 2017;119:661–666.
- Patel HD, Huai K, Elliott N, et al. Differentiating Oncocytic Renal Tumors from Chromophobe RCC using PEER. Eur Urol Open Sci. 2022;46:8–14.
- Radadia KD, Rivera-Núñez Z, Kim S, et al. Accuracy of clinical nodal staging and lymph node dissection. Urol Oncol. 2019;37:577.e17–577.e25.
- Ray S, Dason S, Singer EA. Integrating Surgery in Advanced RCC. Urol Clin North Am. 2023;50:311–323.
- Richard PO, Jewett MAS, Bhatt JR, et al. Active Surveillance for Renal Neoplasms with Oncocytic Features is Safe. J Urol. 2016;195:581–587.
- Rivero JR, De La Cerda J, Wang H, et al. Partial Nephrectomy vs Thermal Ablation for T1 Renal Masses. JVIR. 2018;29:18–29.
- Rodger FE, Brown K, Leung S, et al. Outcomes of biopsy-proven oncocytic neoplasm under surveillance. BJUI Compass. 2022;3:291–297.
- Shuch B, Pantuck AJ, Bernhard J-C, et al. [89Zr]Zr-girentuximab PET–CT imaging of ccRCC: ZIRCON trial. Lancet Oncol. 2024.
- Stillebroer AB, Mulders PFA, Boerman OC, et al. Carbonic Anhydrase IX in RCC. Eur Urol. 2010;58:75–83.
- Thorson D, Bova D, Picken MM, et al. MRI PEER to differentiate chromophobe RCC from oncocytoma. BJUI Compass. 2025;6:e70017.
- Wu Q, Shao H, Zhai W, et al. Molecular imaging of RCCs: ready for prime time. Nat Rev Urol. 2024;1–18.
Conflict of Interest: SD & EAS declare no conflicts of interest related to this manuscript.
Funding: This work was supported by NIH 2P30CA016058-45.



