Submitted - APRIL 04, 2022 | Accepted - OCTOBER 4, 2022 | ePublished - December 31, 2023
https://doi.org/10.52733/KCJ21n3-r3

ABSTRACT
Achieving patient-centered care requires helping patients understand their illness, eliciting patient values, and developing a collaborative care plan with input from patient and physician. Combining existing models in communication skills and shared decision making provides a road map for accomplishing these tasks in delivering patient-centered care. In this article, we highlight the importance of patient understanding of their prognosis as a key step in delivering patient-centered care. We then review literature suggesting that both patient and patient’s physicians’ emotions play an inhibitory role in accurate formulation and communication of prognosis by physicians and accurate incorporation of this information by patients. We postulate that the finding of benefit of early integration of palliative care (PC) in improving patient-centered outcomes may be addressing these inhibitory factors. Key skills of empathic communication by a PC team that is focused on addressing patient emotions may facilitate better understanding of prognosis and thus improved patient-centered decision leading to improved patient centered outcomes. Finally, we propose advances treatment of renal cell carcinoma makes it an ideal disease that can inform this hypothesis of how integration of PC works. Specifically, we propose that the curability potential in metastatic RCC, amplifies challenges associated with patient prognostic understanding and decision making. Studying which discipline – primary oncology team or palliative care team – can help patients achieve more accurate prognostic understanding leading to more patient centered choices and improved patient-centered care.
KEYWORDS
Palliative Care, Renal Cell Carcinoma, Kidney Cancer
INTRODUCTION
“The secret of the care of the patient is caring for the patient.”
- Francis Peabody, 1921
Early integration of palliative care (PC) has been advocated
in routine oncological care in the past decade based
on studies showing improvement in patient symptoms,
quality of life and survival 1-7 . Despite these recommendations,
retrospective review of inpatient and outpatient data
shows that most patients do not receive palliative care services
as recommended by the guidelines, including patients
with kidney cancer 8-10. At the same time, the mechanism by
which improvement in patient centered outcomes including
survival are achieved by integration is not clear.
In the United States, an estimated 79,000 new cases and about 14,000 deaths due to kidney and renal pelvis cancer are projected to occur in 2022 alone11. Over 90% of kidney cancer cases are due to renal cell carcinoma (RCC). About 30% of patients initially present with metastatic RCC and another third of patients will have cancer recurrence with distant metastases after extirpative surgery12,13. With recent advances in immunotherapy, the landscape for treatment and outcome of RCC has changed ushering in multitude of challenges and opportunities14. Here, we focus on one of these challenges, providing accurate prognostic understanding, and the representative opportunity it represents to study the mechanism of palliative care interventions. Advances in treatment has led to additional prognostic uncertainty of “can I be cured?” to the existing prognostic uncertainty of “how long do I have, doctor?” By integrating palliative care into routine RCC care, we propose to study which discipline in the multidisciplinary team can help patients achieve more accurate prognostic understanding, leading to improved decision making and, patient outcomes.
Importance of accurate prognostic understanding
Studies of early palliative care integration demonstrated survival benefits in patients receiving early integration of palliative care5, 15. In one study, at the time of the early integration of PC in metastatic lung cancer, disease was deemed incurable, and yet at baseline, 32% of patients expected that their metastatic disease was curable, and 69% reported that elimination of all cancer was a reasonable goal of treatment. With integration of monthly palliative care visits, a greater percentage of patients in the early palliative care arm were noted to have cultivated an accurate understanding of prognosis (82.5% vs. 59.6%). Furthermore, the authors found that patients having an accurate understanding of disease prognosis and undergoing palliative care treatment were least likely to opt for aggressive and standard of care intravenous chemotherapy treatment within 60 days of death15. The study reported survival benefits in patients with early palliative are arm. It also showed that those with more accurate improved prognostic understanding chose less chemotherapy5,15. Thus, improved, and accurate illness and prognostic understanding and decisions based on accurate prognostic understanding likely play a role in patient outcomes which aligns with our goals of patient-centered care and shared decision making (SDM).
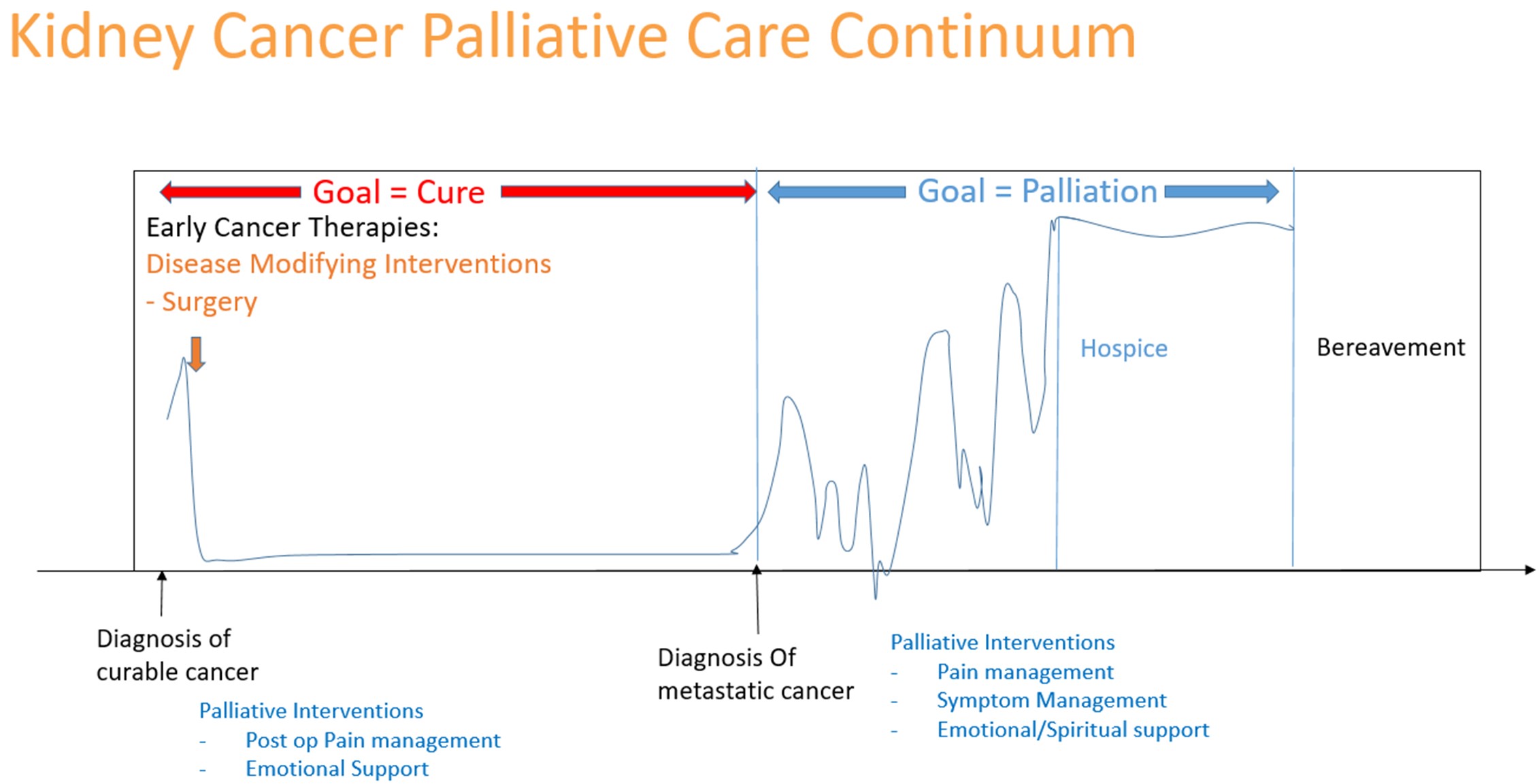
FIGURE 2. Model of palliative interventions in curative and palliative setting for kidney cancer
Model for Conveying Accurate Prognostic Understanding – Communication Skills and Shared Decision Making
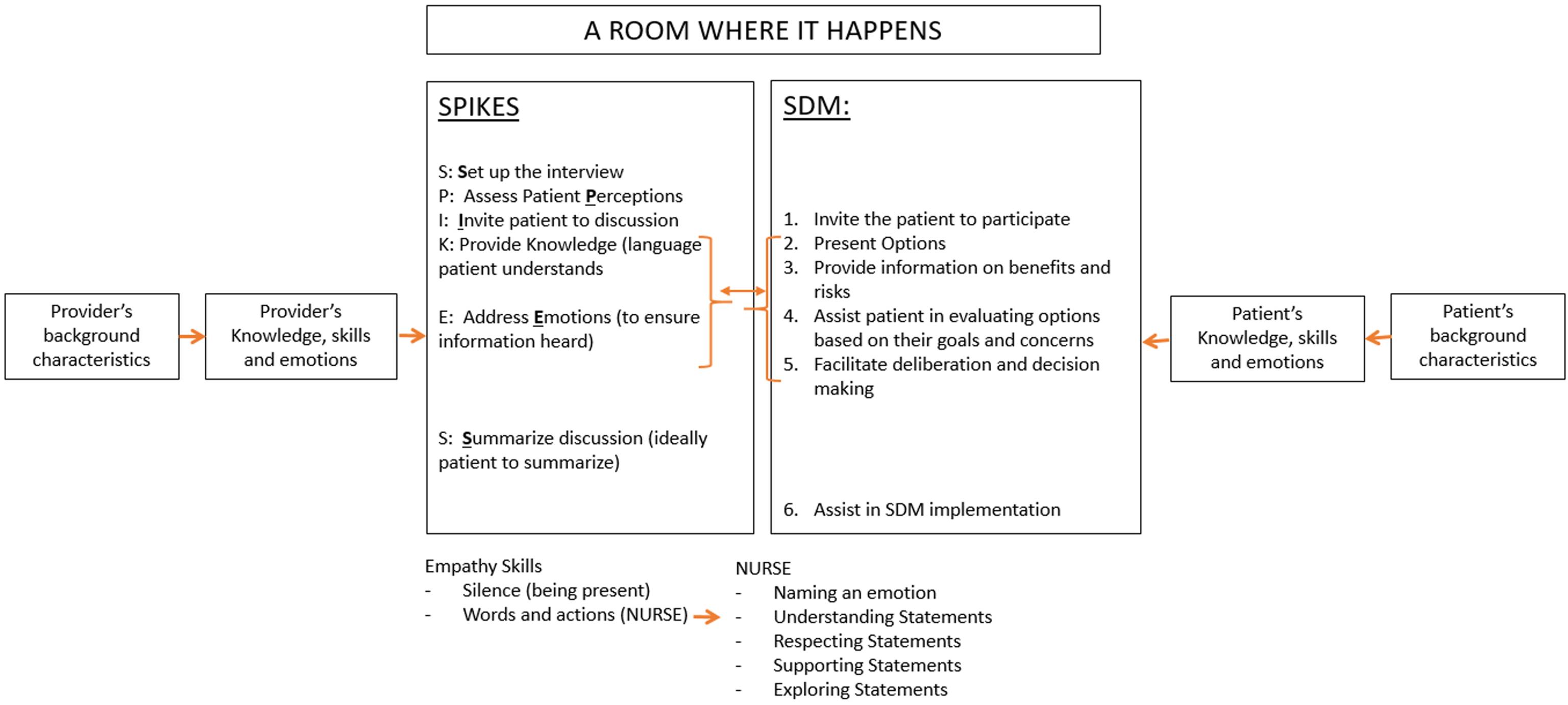
We can view the importance of accurate prognostic understanding in a larger context of patient-centered care. Institute of Medicine defined patient-centered care as “providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions”16. Thus, physicians must accomplish at least two major tasks to provide patient-centered care, 1) to elicit and understand the patient’s preferences, needs, and values and 2) to develop a collaborative plan with the patient that respects and honors their preferences, needs, and values.
There are two separate models that accomplish these
two goals. A communication skills (CS) model, SPIKES, that
provides a roadmap for building rapport, eliciting patient
preferences, needs and values by using skills such as active
listening, reflection, and empathic communication17. A
shared decision making model allows for the development
and implementation of a collaborative plan with input
and collaboration from patients and physicians18. SDM
ensures that among the various treatment choices, patient
preferences and values are guiding the decision. Together,
communication skills and shared decision making provide
specific tasks for physicians and patients to complete to
achieve optimal patient-centered care.
These two tasks can be modeled in a combined CS and
SDM models into one as shown in Figure 1. In this combined
model, when a patient and a physician come together to
make a decision, the SDM model acknowledges that they
both bring their own worldview to the discussion. These
worl`dviews are shaped by individual background, lived
experiences, knowledge, and emotions18. These worldviews
shape the perceptions of the conversation between a patient
and a physician, and the decisions are made based on these
perceptions. These perceptions are what can be assessed by
physicians when listening to a patient’s story initially as they
build a rapport with the patient and family. The language
and vocabulary used by the patient can provide a window
into that patient’s perspectives that will help or impede
future decision making. In addition, the physician needs to
elicit patient preferences and values along with their hopes
and fears by listening and asking direct questions. Physician
uses principles of empathic communication throughout the
conversation and over the long term relationship including
use of open-ended and guided closed-ended questions17.
Once the physician has had a good understanding of the
disease and patient goals and preferences, they can invite the
patient to start the decision-making process for therapies.
The process includes reviewing options for therapies in a
stepwise and iterative manner. For each therapy choice,
risk and benefits are explained and understood and how
they impact patient preferences and goals are highlighted.
Given this can be emotionally challenging and cognitively
overwhelming conversation, the physician needs to conduct
the conversation with great empathy, including using the
non-verbal skills of silence and reflective listening and verbal
skills to ensure patients hear and understand what is said.
Examples of these verbal skills include: Naming an emotion
(N), Understanding statements (U), Respecting statements
(R), Supporting statements (S) and Exploring statements (E)
or commonly referred to as NURSE acronym19.
Although shown in Figure 1 as a series of steps, providing
information is likely to be an iterative process with multiple
pauses, iterations, and restart of the conversation to ensure
that the patient understands their disease, their treatment
goals, and their potential treatment options including risks
and benefits of each of these options. The physician uses
patient’s own words and language to increase the odds
that the patient hears and understands what is being said.
This iterative process allows the physician to guide the
discussion with the patient and families, while eliciting and
refining patient values and preferences. Finally, once all the
discussions have occurred and they can be a collaborative
agreement on best treatment option and specific next steps.
The physician can ask the patient to summarize the patient’s
understanding to ensure all have mutual understanding of
the discussion and the collaborative plan.
Patient and Physician Emotions Are Key
Intermediaries to prognostic understanding
As shown above, to achieve a patient-centered decision,
the physician first must understand the patient worldview
including their goals, values, and preferences, and then
provide information that is heard and understood by the
patient. The information can include prognostic information.
After obtaining a mutual understanding, the physician then
needs to help the patient make decisions that are aligned
with that patient’s goals. The key to this complex process is
the fundamental of CS, empathic communication as shown
in Figure 1.
Both patient and provider emotions play a key role in
what and how information is conveyed and what was heard
during the above conversation. If the conversation or patient
understanding is suboptimal, it may lead to patients making
choices incongruent to their values and preferences. The
challenge thus is both patient and physician emotions.
For example, two separate studies showed potential impact
of physician emotions on formulating and communicating
prognosis. In one study, a longer the physicians had known
the patient, more likely the physician would err in their
prognostication [20]. In a different study, what physicians
told the researchers about prognosis (formulated prognosis)
was and what they told patients (communicated prognosis)
differed by more than 20% and both were significantly
inaccurate (for example, communicated 90 days survival
estimate when actual was 26 days)20, 21. Thus, both conscious
and unconscious optimism, possibly from provider emotions,
plays a role in formulation and communication of inaccurate
prognosis21.
Similarly, patients’ emotions and world view may
impact what they hear and how they make decisions. Aim
of phase 1 studies is to assess for dose limiting toxicities
and optimal dose for future research and involve first in
human drug or combination of drugs. Review of informed
consents have shown that there is almost never a promise of
direct benefit to subjects, rarely mention cure, and usually
communicate seriousness and unpredictability of risk22.
Despite their consent, patients participating in these trials reported a different perception and that provides insights into how patients perceive and make decisions. In a large multi-centered study of one hundred-sixty-three patients participating in phase 1 studies showed that 75% of patients felt the pressure to participate because their cancer was growing and similar percentage of patients reported feeling somewhat or very anxious when they were not receiving some sort of anti-cancer therapies [23]. More interestingly, only 3% of participants reported they personally were very or somewhat unlikely to benefit from participating in the phase 1 study even though 60% of them estimated that others were unlikely to benefit23.
In a different study of patients being evaluated for phase 1
studies showed that those patients who enrolled in the phase
1 study reported higher likelihood of response to therapy
compared to patients that did not enroll or physicians who
had consulted with them24. Thus, patients perceive and
process information thru the lens of their emotions and
worldview which may lead to more inaccurate expectations
of benefit of therapy.
Thus, physician and patient emotions can prevent
accurate prognostication and communication of the prognosis
by the physician and can lead to patients making decisions
without accurately understanding of their prognosis and its
implications on their therapy options and likely outcomes.
Thus, a decision made with inaccurate information can lead
to flawed and ultimately poor decisions such as continuing
ineffective therapies or taking therapies that are unlikely to
benefit and may even be counterintuitive to their stated goals.
Integration of Palliative Care in RCC and Exploration of Mechanism of action of Palliative care
Palliative care is specialized medical care delivered by a multidisciplinary team of physicians, nurses, social workers, and other specialists addressing multiple domains of care.25, 26. Palliative care team focuses on symptom management as well as provides expert communications with patients and caregivers. The expert communication, as shown in the Figure 1, involves addressing emotions with empathy. When symptom management and expert communication are provided by the primary oncology team, it is called “primary palliative care” and when using a subspecialty team, it is called “subspecialty palliative care”27. Post-operative pain by the urologist; prevention and treatment of side effects of medical therapies by the medical oncologists; radiation to alleviate pain from bone metastasis by the radiation oncologists are all examples of delivery of primary palliative care delivered by the oncology team. In addition to these symptoms, one or more of the primary teams can discuss treatment goals and address patient emotional and spiritual needs. When needed, these primary teams can consult with subspecialists to help them manage patient’s symptoms or communications, it would be considered specialist palliative care. Using this definition, we can conclude that palliative interventions start concurrently with curative treatments, continue alongside palliative intent therapies, until a point where focus changes to providing comfort, eventually transitions to hospice (Figure 2).
All the challenges to SDM listed above with inaccurate
prognosis, communication, and patient perceptions have
been studied prior to advances in oncologic therapies such
as immunotherapy. Immunotherapy, and specifically
immune checkpoint inhibitor (ICI) therapy, has changed the
landscape of management of RCC. Prior to the advances in
immunotherapy, the answer to the question “can I be cured”
when presenting with metastatic disease was “no” with
confidence ad now, it is much more nuanced. Recent phase
III studies with combination of immunotherapies show that
even with metastatic disease, up to 7-16% patients can have
long-term complete remission and may be even cured28-31.
This creates a further challenge and an opportunity in
communicating prognosis to achieve patient centered
decision using SDM.
This challenge of difficulty in communicating
‘curability’ highlighted in a study of patients with advanced
lung cancer and genitourinary (GU) malignancies receiving
immunotherapy32, 33. Approximately 20-95% of patients
had an inaccurate understanding of their curability and
had increased anxiety compared to those with an accurate
understanding of their cancer34.
Considering the challenge of prognostic uncertainty
caused by improved RCC outcomes and the observation that
palliative care integration has been shown to both improve
prognostic understanding and contribute to the making of
more patient-centered decisions, RCC is an ideal disease in
which to study how palliative care improves patient survival.
There is already pilot data of integration of palliative
care into routine RCC care in the immunotherapy era27. We
hypothesize that using the model for decision making above
and understanding how the above tasks are completed,
we may be able to understand the mechanism by which
integration of palliative care enhances patient outcomes. We
further hypothesize that the advances in RCC treatment in
the past decade with increased uncertainty makes it an ideal
disease to study and elucidate these mechanisms that can
then be utilized in other diseases.
Mechanisms include improved patient prognostic
understanding via improved management of patient emotions
and communication. As studies have showed that the longer
an oncologist knows a patient, accurate prognostication
becomes more difficult, and it becomes even harder to
communicate this prognosis accurately, an independent
palliative team may have less emotional burden to facilitate
an honest conversation20, 21. A separate team that is focused
solely on patient symptoms including emotional symptoms,
also allows patients increased opportunities to feel “cared
for,” as was highlighted by Dr. Peabody, without getting
chemotherapy and scans.
We hypothesize that potential mechanisms of the benefits
from palliative care may include:
• Improved illness communication, through improved physician understanding of patient worldview and management of patient emotions
• Improved prognostic understanding leading to improved shared decision making
Patients with RCC undergoing concurrent oncological
and palliative care can be assessed along with each team for
how information is conveyed and heard by the patient. While
both the primary oncology team providing palliative care
can be skilled, the context of the conversations with patients
who are focused on cancer and therapies may preclude
accurate exchange of information due to the emotional
reactions from both patients and the primary team.
Having a subspecialty palliative care team with expertise
in symptom management and communication skills may
allow patients and the PC team to have discussions in a
non-cancer treatment context, which may facilitate better
information incorporation and even improved decision
making.
By evaluating how information on diagnosis,
staging and treatment goals are discussed, how patient
understands them and how the discussion of prognosis is
conducted, and decision made to start, continue, change, or
stop cancer directed therapies will allow us to understand
the role primary oncology and palliative care team plays
in improving patient understanding and decision making.
An improved mechanistic understanding of how
palliative care team impacts patient outcomes may help
guide future implementation and research. Understanding
whether the primary team, due to its relationship with
the patient, is likely to be handicapped in an objective
discussion may facilitate better identification of when and
how to integrate palliative care. Understanding which
factors predict which patients view and relate to primary
team and the palliative care teams different may also
provide better insights into which patients need early
palliative care integration to optimize patient-centered
care.
REFERENCE
# Corresponding Author: Biren Saraiya, MD
Division of Medical Oncology, Department of Medicine,
Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood
Johnson Medical School, New Brunswick, NJ 08901