Submitted - JUN 11, 2023 | Accepted - AUG 31, 2023 | ePublished - OCT 10, 2023
https://doi.org/10.52733/KCJ21n3-r1
ABSTRACT
Cytoreductive nephrectomy (CN), or the removal of the primary kidney tumor in the setting of metastatic disease, plays a critical role in the treatment of metastatic renal cell carcinoma (mRCC). The benefits of CN, are multifactorial including alleviating symptoms but also eliminating cells potentially prone to future metastasis, and potentially extending a patient's survival. As innovations in mRCC treatment continue to emerge, the importance and timing of CN in patient care remains the subject of ongoing debate in the scientific community. With advancements in modern therapies and the introduction of immune checkpoint inhibitors (ICI), the optimal integration of CN in mRCC management becomes even more important to investigate. This manuscript reviews the key literature related to CN and critically evaluates data that investigated CN efficacy. Furthermore, this article summarizes data to help identify ideal candidates for CN, and explores options for integrating CN within the contemporary systemic therapy landscape.
KEYWORDS
Cytoreductive nephrectomy, Immune Checkpoint Inhibitors, Renal
Cell Carcinoma, Patient Selection.
INTRODUCTION
Renal cell carcinoma will affect about 82,000 people in the U.S. in 2023. Unfortunately, around 30% of the individuals who present with RCC will have metastatic disease either within their regional lymph nodes or at distant sites at the time of their presentation 1,2. While the majority of patients with metastatic RCC are not curable, there has been a consistent improvement in the overall survival of patients who develop mRCC over the last two decades 3. Much of this improvement has come from a deeper understanding of RCC tumor biology, and the host immune response within the tumor microenvironment4. One of the most important advancements in mRCC management has been the development of immune checkpoint inhibitor therapy 5-9, which has led to a substantial improvement in survival for mRCC patients compared to single agent tyrosine kinase inhibitor (TKI) therapies. As a result, standard first line therapies for mRCC are combinations of ICI/ICI or ICI/TKI therapies.
While there have been
significant improvements in the
survival of patients with mRCC due
to advancements in systemic therapy,
surgery continues to remain a critical
component of the management of a
subset of patients with mRCC. CN
has been used throughout the history
of mRCC management, but became
standard of care in 2001 based on the
results of two randomized trials 10-12.
Cytoreductive nephrectomy is defined
as the removal of the primary renal
mass in the setting of synchronous
metastatic disease 13. This can either
occur prior to the receipt of any systemic
therapy (termed “upfront” CN) or after
systemic therapy has been delivered
(termed “deferred” CN). There are
multiple reasons that CN is performed:
1) to remove tumor that harbors cells
capable of metastasizing or are resistant
to therapy, 2) to palliate symptoms such
as pain, gross hematuria, early satiety,
which thereby improves the patient
quality of life, and 3) to extend patient
survival. Despite these indications, the
role of CN has become controversial due
to publication of a randomized trial in
2018 that demonstrated non-inferior
outcomes for CN combined with
sunitinib compared to sunitinib alone14.
This clinical trial was controversial
and had significant limitations, which
reduced the impact of the findings in the
context of modern mRCC management.
The goal of this review is to concisely
summarize the historical context of
CN leading up to the current era of ICI
therapy, including a critical analysis
of the controversies surrounding CN
and how CN can best be incorporated
into the management of patients with
mRCC.
CYTOREDUCTIVE NEPHRECTOMY – A BRIEF HISTORY
Prior to the implementation of effective systemic therapies, CN was used sparingly and was considered more for symptomatic purposes. Spontaneous regression of metastatic disease after patients received CN was reported but exceptionally rare15. Cytoreductive nephrectomy became a standard of care after the publication of two clinical trials in 2001: SWOG 8949 and EORTC 3094710,12. The two trials had similar study designs and randomized patients to either IFN-α alone or upfront CN followed by IFN-α. A combined analysis of these trials demonstrated an overall survival benefit favoring the CN arm (13.6 months vs 7.8 months, P=0.001)11. While these data are older, and IFN-α is significantly less effective than modern ICI therapy, the data from these trials provide a unique view of the benefit of CN. When these trials were conducted, there were no approved second line systemic therapy options available. Therefore, the survival data from these trials is less influenced by subsequent therapies that patients might have pursued outside the trial setting. This offers a clearer understanding of the impact of CN on overall survival, devoid of the effects created by different second line therapies on patient survival. These data demonstrate a significant benefit for appropriately selected patients undergoing CN.
The cytokine era of systemic therapy (prior to 2006) consisted of IFN-α and IL-2, both of which had limited efficacy and high toxicity16.After the cytokine era of systemic therapy, TKI therapy became standard of care starting with sorafenib and sunitinib therapy, after two phase III trials in 2007 demonstrated benefit of these agents over IFN-α 17,18. In 2015, nivolumab (an anti-PD1 antibody that activates exhausted CD8+ T cells) became the first FDA approved ICI therapy for the treatment of mRCC, bringing about the ICI therapy era of mRCC management19. Since that time, multiple phase III trials have demonstrated the ability of ICI therapy to extend patient survival in the setting of mRCC. For example, the phase III trial CheckMate 214 published extended follow-up showing a median overall survival of 56 months for patients treated with nivolumab plus ipilimumab, and the KEYNOTE-426 trial demonstrated a median overall survival of 46 months among patients treated with pembrolizumab plus axitinib20,21. These results are nearly two fold higher than the median overall survival of patients receiving sunitinib, which was 26 months upon the trial's final analysis22. Thus, there has been a clear improvement in the survival of patients with mRCC being treated in clinical trials with modern ICI therapies.
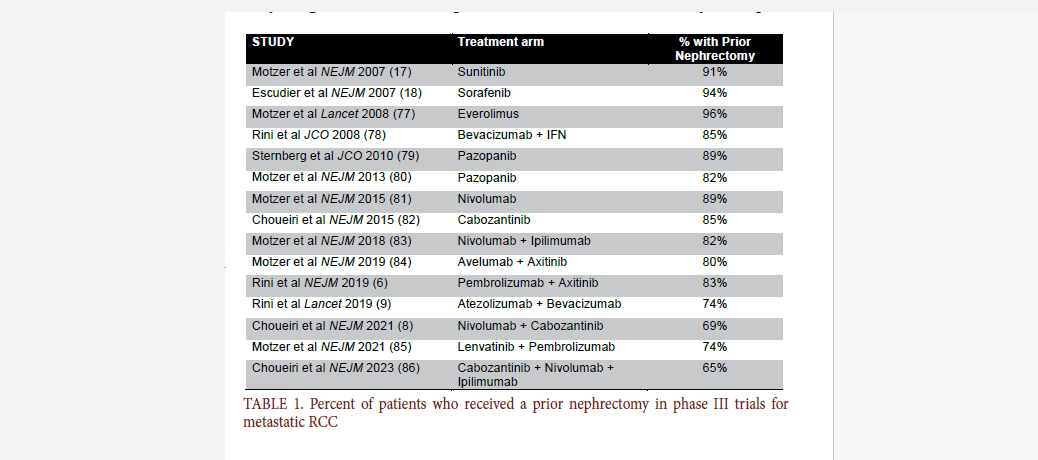
It is important to note that all of
the phase III trials investigating modern
systemic therapies for mRCC included
a large proportion of patients that had
received a prior nephrectomy (either
prior to metastatic progression or at the
time of synchronous metastatic disease)
(TABLE 1). Thus, the survival benefits
of all modern systemic therapies for
mRCC have to be interpreted knowing
that most patients had their primary
tumors removed prior to systemic
therapy administration. In truth,
randomized clinical trial data for
systemic therapies in mRCC do not exist
in the absence of surgery, which is a key
reason that surgery is considered part
of the multidisciplinary care of mRCC.
CONTROVERSIES REGARDING CYTOREDUCTIVE NEPHRECTOMY
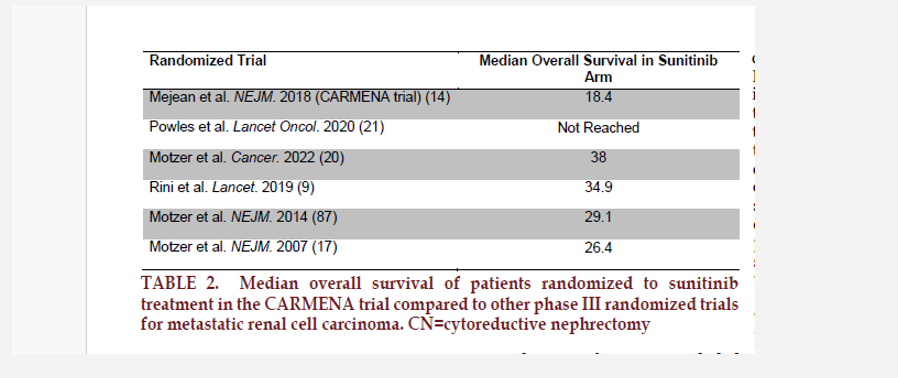
The most recent catalyst for CN controversy was publication of the results of the CARMENA (Cancer du Rein Metastatique Nephrectomie et Antiangiogéniques) clinical trial14, randomized 1:1 mRCC patients treated with upfront CN followed by sunitinib versus sunitinib alone. This was designed as a non-inferiority trial with overall survival as the primary endpoint and statistically powered to include 576 patients. The trial was published in 2018 and demonstrated non-inferior survival outcomes in the systemic therapy alone arm vs CN plus systemic therapy arm (18.4 vs 13.9 months, respectively). The results and trial design sparked immediate debate in the literature and at scientific conferences. Despite providing the first randomized clinical trial data in two decades, the CARMENA study had significant limitations. First, the trial enrolled extremely slowly and did not reach its accrual goal. Two planned interim analyses (after 152 and 304 deaths) were performed and both concluded that the trial should cont inue. However, immediately after the second interim analysis, the sponsor closed the trial because of poor accrual. At the time of publicat ion, the tr ial was able to enroll 450 patients across 79 centers over 8 years, significantly short of enrollment goal of 576 patients. In both study cohorts, there was significant cont amination from not receiving the primary treatment or receiving other seconda ry treatments, which could bias the outcomes.
The trial was analyzed according
to the intention-to -treat principle, but
patients were frequently managed
differently than their designated trial
ar m protocol. Seven percent of patients
in the surgic alarm did not receive a
CN and 18% of patients did not receive
subsequent sunitinib therapy and 5% did
not get sunitinib. In both groups, about
half of patients received additional l ines
of systemic therapies after sunitinib.
One of the strongest crit icisms of t his
study was the enrich ment of the study
c ohort for poor risk patients with
high volume metastatic disease. In
CARMENA, the median pat ient had 2
sites of metastatic disease w ith 14 cm
of overall tumor burden with 8.8 cm
primary tumors. Nearly half (44%) of
patients enrolled in the CN arm had poor
risk disea se according to the Memor ial
Sloa n Ketter ing Cancer Center (MSKCC)
mRCC risk classi ficat ion. Multiple pr ior
retrospective studies have demonstrated
that poor risk patients with high volume
disease outside of the kidney are least
likely to derive a survival benefit from CN
and should be counseled against upfront
surgery. Evaluat ion of the CARMENA
patients and known predictors of poor
outc omes af ter C N demonstrate a highrisk
patient population enrolled in the
study to receive CN. The MD Anderson
Cancer Center investigators published
preoperative predictors of worse overall
survival after CN23. These pr edictors
included node positive disea se (N+),
bone metastases, and high stage disease
(clinical T4 disease). The CARMENA
patients included 35% with N+ disease
and 36% with bone metastases.
Additionally, 70% within t he surgery
arm had cT3-T4 disea se compared
to only 51% within the sunitinib only
arm. The select ion of high-risk patients
for inclusion in this trial is further
supported by the fact that the median
overa ll sur vival in the sunitinib ar m is
much lower than the median survival
in the sunitinib arm from other modern
phase III randomized
trials (TABLE 2). A
post hoc analysis of
the CARMENA trial
demonstrated that
patients with one
IMDC risk factor had
significantly longer OS
in comparison to those
with two or more IMDC
risk factors 24. Lastly,
it should be noted that
systemic therapy options
evolved considerably
during the eightyear
study and when
the trial results were
published, sunitinib
was no longer used for
first line therapy for
mRCC patients, further
limiting the applicability
of the results to modern
clinical practice. Strong
conclusions from the
CARMENA trial should
be that appropriate
patient selection is
critical for successful
outcomes25.
A n o t h e r question that was attempted to be investigated with a randomized clinical trial is optimal timing of CN (before or after systemic therapy). The SURTIME trial (Immediate Surgery or Surgery After Sunitinib Malate in Treating Patients with Metastatic Kidney Cancer) investigated the timing of CN and sunitinib therapy26. Patients were randomized to either upfront CN followed by sunitinib or sunitinib therapy followed by deferred CN. Like CARMENA, SURTIME had difficulty enrolling patients and only 99 patients were recruited to the trial before it was closed. In the intention to treat population, the 28- week progression free rate (PFR) was 42% compared to 43% in the upfront versus deferred CN patients (P=0.61) and the median overall survival was 15 months versus 32.4 months in the upfront versus deferred CN patients (P=0.03) 26. The trial indicated no significant improvement in the 28-week PFR with a possible survival benefit for deferred CN but results are difficult to interpret with small patient numbers. As a response to poor enrollment, 28- week PFR became a revised primary endpoint. Additionally, within the deferred CN arm, 29% of patients did not undergo surgery while 92% of patients in the upfront CN received surgery. The trial was not powered to detect an overall survival benefit and the survival analysis was exploratory. A per-protocol analysis ultimately did not demonstrate a significant overall survival difference between the two arms. Lastly, sunitinib as first line therapy is no longer clinically applicable to modern management of mRCC. In summary, the SURTIME trial suggested minimal difference in endpoints with different timing of CN but did not definitively answer the question.
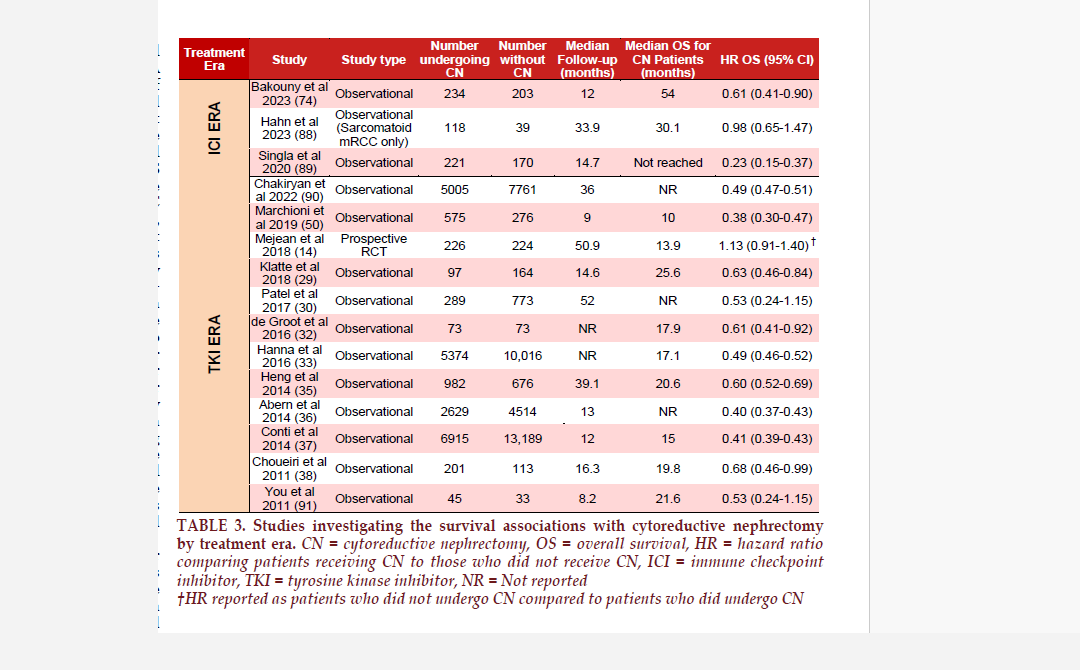
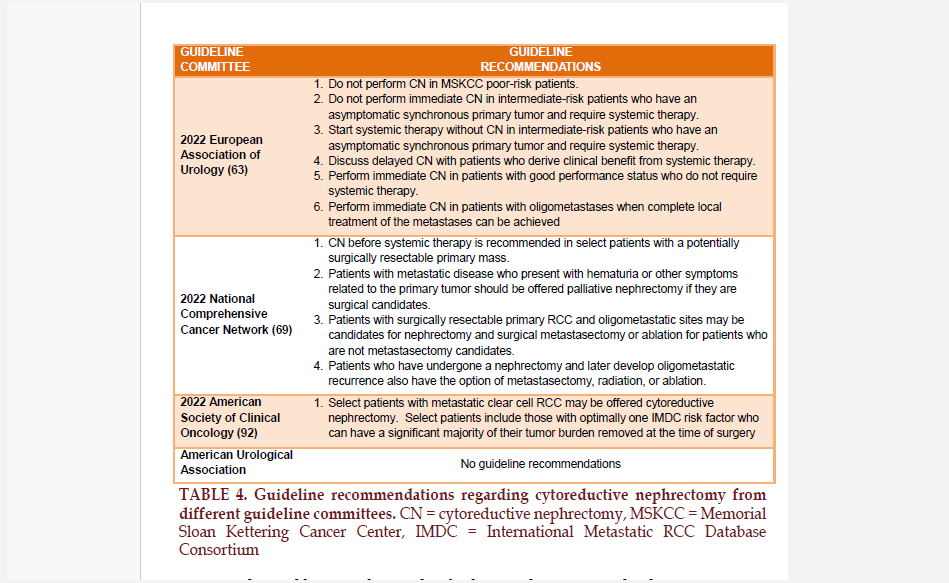
The CARMENA and SURTIME trials fueled significant controversy regarding the utility and timing of CN in the management of patients with mRCC. Following the publication of these trials, the European Association of Urology (EAU) guidelines regarding CN were modified and recommended poor risk patients (based on MSKCC risk criteria) should not undergo CN and intermediate and poor risk patients should receive systemic therapy first before CN is considered27. The findings of these clinical trials, however, need to be balanced with the large number of observational data that suggest a continued survival benefit for patients receiving CN (TABLE 3) 328- 38. The conflicting evidence between randomized trials and observational studies likely resides in surgical selection bias. The appropriate selection of patients for CN is critical to successful outcomes, and this concept is reflected in many modern guideline recommendations (TABLE 4).
PATIENT SELECTION FOR CYTOREDUCTIVE NEPHRECTOMY – CHOOSING WISELY
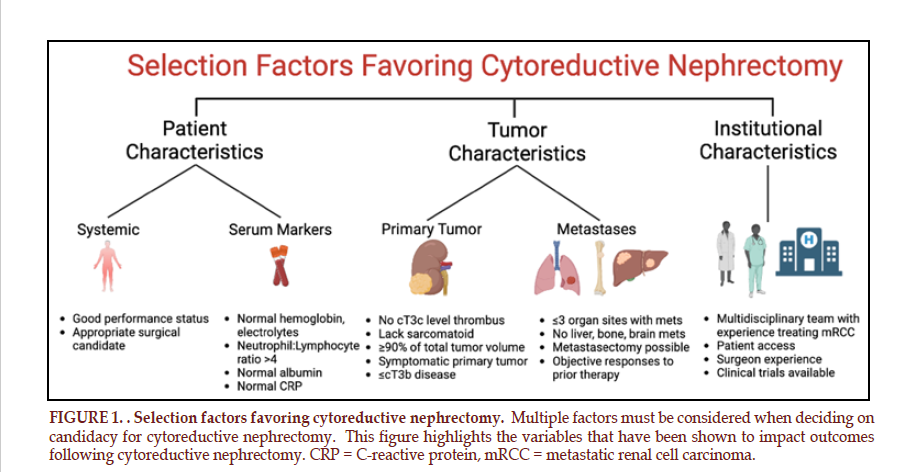
There are no standardized selection factors for identifying ideal patients for CN. Multiple different prognostic and predictive variables have been identified, all of which have been investigated in observational studies. In general, variables that predict survival outcomes following CN fall into three major categories: institutional associated variables, patient associated variables, and tumor associated variables. Within each of these categories, multiple variables have been identified that help to select ideal candidates for CN (FIGURE 1).
Tumor Characteristics
Certain characteristics of the primary and metastatic tumors are significantly associated with outcomes following CN. Patients are thought to be more likely to benefit from CN if the primary tumor accounts for the majority of total tumor burden within the patient 39, 40. One study demonstrated that when assessing both metastatic and primary tumors, if the volume of the primary tumor comprises more than 90% of the total tumor burden, patients are likely to experience improved cancer-specific survival following CN 40.
Also, primary tumors with
a tumor thrombus pose a unique
challenge in the metastatic setting.
Tumors that invade the inferior vena
cava can progress rapidly toward the
right atrium and cause significant
symptoms such as leg swelling, fatigue,
weight loss, liver failure and ultimately
death. Up to 50% of patients with tumor
thrombi can have metastatic disease.
Abel et al. demonstrated that compared
to tumor thrombi that only invade
the renal vein (i.e., level 0), tumor
thrombi that have advanced above the
diaphragm (level IV) have significantly
reduced overall survival (median 22 vs
9 months, respectively)41. Conversely,
tumor thrombi that are still below the
diaphragm but above the renal vein did
not have significantly worse survival
than level 0 thrombi (20 vs 22 months,
respectively) 41. Thus, patients with
tumor thrombi invading the IVC should
still be considered for CN by experienced
surgeons.
The number and location of metastases should also be considered when identifying CN candidates. A greater number of different metastatic sites is associated with inferior outcomes following CN and certain locations portend more aggressive disease42-45. Patients with lung, pancreas, thyroid, or adrenal metastases tend to have a more indolent pattern of progression and may be better suited for upfront CN, while patients with liver or brain metastases tend to have worse overall survival and more rapid disease progression and may benefit from upfront systemic therapy followed by deferred CN in those who respond or demonstrate disease stability42-45. Metastasectomy should also be considered particularly for patients with oligometastatic disease in surgically resectable locations. Patients undergoing complete metastasectomy with CN (either at the same time or in a delayed fashion) have superior cancer-specific survival; however, patients undergoing metastasectomy typically are highly selected for excellent performance status and more indolent tumor biology46,47. If surgical extirpation is not an option, metastasis directed therapy can be achieved in some circumstances using either ablative technology48. or stereotactic body radiotherapy (SBRT). A phase 2 trial by Tang et al. recently reported treating 30 patients with ≤5 metastatic tumors with SBRT to all metastatic sites. Median progression-free survival was 22.7 months and authors concluded that SBRT may delay systemic therapy initiation or facilitate breaks from systemic therapy among patients with oligometastatic RCC49.

Additional tumor related characteristics that should be considered when deciding on CN are tumor associated symptoms, tumor histology, and sarcomatoid dedifferentiation. Patients may present with a symptomatic primary tumor with pain, gross hematuria, or paraneoplastic syndromes. In these situations, CN should be considered for appropriate surgical candidates to palliate symptoms and improve patient quality of life. Regarding non-clear cell histology, outcomes following CN are less well defined, but in general similar principles apply to patient selection and observational studies have demonstrated a survival benefit for patients receiving CN even with non-clear cell histologies50-51. Tumors harboring sarcomatoid dedifferentiation are particularly aggressive. Prior to ICI therapy, patients with metastatic sarcomatoid RCC often had rapid disease progression and short median overall survival, and observational studies of CN for patients with metastatic sarcomatoid disease showed worse survival compared to patients without sarcomatoid disease52. Sarcomatoid disease appears uniquely responsive to ICI therapy, however, and patients with sarcomatoid disease have experienced impressive responses with ICI therapy compared to older systemic therapy agents. The KEYNOTE-426 trial evaluating pembrolizumab+axitinib and the CheckMate 214 trial evaluating nivolumab+ipilimumab both demonstrated improved disease response among sarcomatoid tumors compared to the sunitinib control arm5,6. Thus, patients with sarcomatoid dedifferentiation and mRCC should be considered for upfront ICI/ICI or ICI+TKI therapy and later treated with surgery if there has been significant response to systemic therapy and a residual primary tumor. One challenge with sarcomatoid dedifferentiation is that clinicians frequently do not know if the tumor harbors sarcomatoid dedifferentiation at presentation or prior to offering surgery as it is not reliably detected on imaging or biopsy and is mainly determined after nephrectomy has been performed.
Among patients with borderline
unfavorable tumor characteristics,
some propose using upfront systemic
therapy as a “litmus test” to determine
whether or not the patient will progress
even in the setting of systemic therapy.
If a patient progresses, they are unlikely
to benefit from surgical intervention.
However, if a patient has a durable
response to therapy, they may be more
likely to benefit from surgery. In these
situations, CN can be considered in the
deferred setting. This is particularly
relevant in the ICI therapy era, where
significant responses to ICI/ICI and
ICI/TKI therapy have been observed.
Patient Characteristics
One of the fundamental challenges faced by clinicians is determining the fitness of patients preoperatively and estimating a patient’s individual risk of morbidity and mortality for a complex operation such as CN. Various measures of performance status have been used to estimate these risks including the Eastern cooperative group performance status scale53, Karnofsky performance status54, and Charlson comorbidity index55. While each of these measures can give a general idea of the patient level of fitness and comorbidity, none were specifically designed to measure a patient’s risk of morbidity from CN or their subsequent survival following CN. In general, patients with poor performance status are felt to be higherrisk candidates for CN and favored to receive initial systemic therapy. Patient performance status is dynamic, however, and may improve after receiving systemic therapy making them eligible for CN after initial systemic therapy. This demonstrates the importance of a multidisciplinary approach to mRCC patient management when determining surgical eligibility, which should be considered not only during the initial evaluation of the patient but throughout a patient’s disease course. Other serum-based markers have been identified as predictive of patient outcomes. The presence of preoperative anemia, hypercalcemia, and hypoalbuminemia have been associated with worse survival following CN56,57. Markers of systemic inflammation such as the elevated neutrophil lymphocyte ratio and elevated C-reactive protein have also been associated with worse survival outcomes following CN58- 60. While each of these variables may incrementally better inform selection of patients for CN, none has been routinely incorporated into patient selection and most require further external validation. Additionally, the majority of these markers were evaluated in the TKI therapy era, and require further study in the setting of modern ICI therapy.
Prognostic scores
Various prognostic scores have also been developed that incorporate many of the previously described variables. Two frequently used prognostic scoring systems are the Memorial Sloan Kettering Cancer Center (MSKCC) risk criteria and the International Metastatic RCC Database Consortium risk criteria61,62. The MSKCC and IMDC risk criteria are similarly designed but incorporate different prognostic variables that predict survival outcomes for patients with mRCC. Currently, the IMDC risk criteria are more frequently utilized as they were more recently developed in the TKI therapy era. Each variable in the IMDC risk criteria is assigned 1 point and the variables included are neutrophilia, thrombocytopenia, anemia, hypercalcemia, Karnofsky performance status <80, and time from diagnosis to systemic therapy of <1 year. Patients with mRCC are categorized into favorable (0 risk factors), intermediate (1-2 risk factors) and poor (≥3 risk factors) risk groups. The EAU guidelines recommend that intermediate and poor risk patients should receive systemic therapy first and poor risk patients do not benefit from CN63 The limitation of using these risk stratifications to make decisions regarding CN is that they were not designed specifically to address survival outcomes following CN. Also, the risk classifications are often dynamic and may change during the disease course. A patient may initially present with poor risk disease (due to lab abnormalities such as anemia, hypercalcemia, and neutrophilia) but these may improve after receipt of systemic therapy or CN64,65. In order to address these limitations, prognostic scoring systems have been developed specifically in CN patient populations to help identify appropriate candidates for CN23,66 Updating their prior prognostic classification system66, the MD Anderson Cancer Center group recently evaluated a modern cohort of CN patients and identified 9 predictors of worse overall survival following CN23. The advantage of this study is that it incorporates variables that can be obtained preoperatively to risk stratify patients and was designed specifically in a CN patient population. Similarly, a study using the European registry for metastatic RCC (REMARCC) developed a scoring system to predict overall survival following upfront CN. The study incorporated BMI, metastatic location (lung, liver, bone), number of metastatic sites, and performance status into their model for predicting survival67. Both studies require further external validation and given the time periods within which patients were included, it is unlikely that many patients received ICI therapy during the course of their mRCC treatment, highlighting the need for prospective registries of mRCC patients receiving CN to identify predictors of favorable outcomes.
The medical system impact on cytoreductive nephrectomy
Another critical aspect of outcomes following CN is the system in which the patient is treated. Management of patients with mRCC is nuanced and complex, requiring coordination between multiple disciplines. Patients with mRCC interact with oncologists (including urologic, medical and radiation), pathologists, radiologists, interventional radiologists, anesthesiologists, nursing staff (in the clinic, infusion centers, inpatient units, research coordinators, and operating room), medical technologists (in the operating room and clinics), phlebotomists, billing and insurance staff, fellows, residents, and medical students to name only a few. Coordination of these components requires a system designed to and experienced in delivering care to patients with mRCC. Poor access to centers such as these may limit the ability for a patient to receive CN and negatively impact the survival outcomes of patients following CN. Cytoreductive nephrectomy has been shown to be more frequently performed at academic institutions and among the privately insured30. Higher hospital volume is also independently associated with improved mortality following CN68. Thus, patient access to systems that routinely manage mRCC and a thoughtful multidisciplinary discussion of these complex cases is critical for favorable outcomes.
CYTOREDUCTIVE NEPHRECTOMY IN THE ERA OF IMMUNE CHECKPOINT INHIBITORS
Since nivolumab approval in 2015, there has been rapid incorporation of ICI therapy into the management of mRCC, and ICI/ICI or ICI/TKI combinations are now first line therapy69. The improvements in response rates to modern systemic therapy again begs the question if there is still a role for CN. Given ICI therapy’s relatively recent approval, very few studies have addressed the impact of CN on survival outcomes in the setting of ICI therapy and those that have are often small sample sizes with limited follow-up70-73. Cytoreductive nephrectomy following ICI therapy does appear safe and feasible. One of the largest multi-institutional studies by Shapiro et al. demonstrated that among 75 patients undergoing deferred CN following ICI therapy, the high-grade complication rate was only 3% with no 90-day mortalities. Additionally, 48% of patients were able to enter a period of surveillance following their CN, delaying further systemic therapy 71. Thus, patients being treated with CN at experienced centers face low morbidity rates even compared to historic CN series57.
Regarding survival outcomes, a
recent study by Bakouny et al used the
IMDC database to evaluate the impact of
upfront CN (N=234) vs no CN (N=203)
on survival outcomes among patients
treated with ICI therapy. Multivariable
analysis demonstrated upfront CN was
associated with significantly improved
overall survival compared to no CN
among patients treated with ICI therapy
(HR 0.61, 95% CI 0.41-0.9, P=0.013)74.
These studies again appear to confirm
that among appropriately selected
patients, CN is safe and associated with
improved survival.
CYTOREDUCTIVE NEPHRECTOMY FUTURE DIRECTIONS
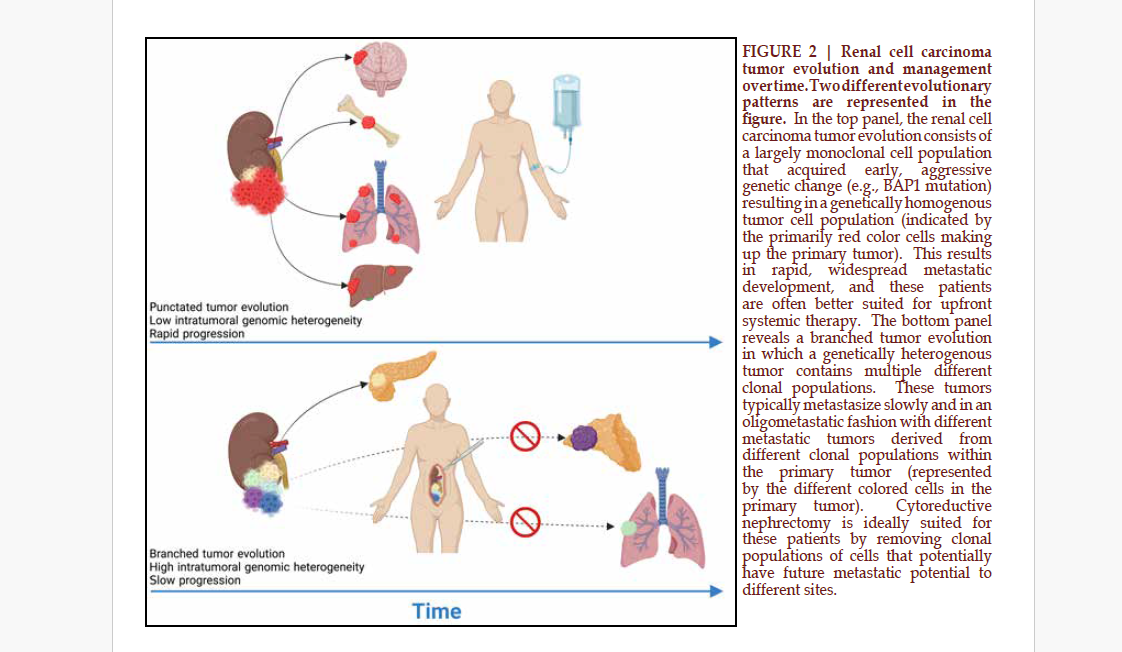
As we gain a deeper understanding of RCC tumor biology, we may begin to better select patients for CN based on tumor biology. The TRACERx studies have demonstrated that tumors harboring BAP1 mutations are associated with rapid tumor progression and low intratumoral genomic heterogeneity. These patients may not derive a survival benefit from CN compared to tumors harboring primarily PBRM1 mutations without BAP1 mutations, which are associated with slow progression and high intratumoral genomic heterogeneity (FIGURE 2)75. The Memorial Sloan Kettering group also demonstrated that BAP1 mutations negatively affected OS among patients undergoing CN, while SETD2 and KDM5C mutations were associated with reduced risk of death76. Additional explorations into the tumor and immune microenvironments may help identify predictive biomarkers associated with patient survival following CN4.
Clinical trials investigating CN
are currently being conducted. Active
trials include PROBE (NCT04510597),
NORDIC-SUN (NCT03977571), and
Cyto-KIK (NCT04322955). While these
trials will provide insight on the role
of CN in the deferred setting, there are
currently no large trials investigating
the use of upfront CN, which is utilized
in healthy patients with minimal
metastatic disease. Prior studies
including CARMENA and SURTIME
have demonstrated the difficulties
accruing to CN specific trials, thus
other mechanisms for studying CN
in a robust and generalizable manner
are necessary to supplement clinical
trials. An additional robust method for
studying CN in the future will be multiinstitutional
prospective registries to
investigate CN outcomes, particularly
in the upfront setting. While not
randomized, prospective registry data
can still provide important insight into
CN practice patterns, perioperative
morbidity, and survival outcomes,
particularly in the rapidly changing
treatment landscape of mRCC.
An additional unexplored
area of research is the study of patient
reported outcomes and quality of life
following CN using validated HRQoL
instruments used in most studies of
systemic therapy. One of the primary
proposed benefits of CN is that it
improves patient symptoms and quality
of life, but evidence to support this
hypothesis is absent. Additionally, it
is critical to involve multidisciplinary
care across the patient’s journey of
treatment. Future studies to address
these issues must be conducted.
CONCLUSION
Cytoreductive nephrectomy remains a critically important component of the multidisciplinary approach to management of patients with mRCC. A large body of evidence supports the use of CN in appropriately selected patients. Patients with good performance status and limited metastatic burden are ideal candidates for CN. The use and timing of CN will continue to evolve as our understanding of RCC tumor biology advances and systemic therapies continue to improve.
ACKNOWLEDGMENTS
Figures were constructed using biorender.com.
FUNDING The authors report no funding.
AUTHOR CONTRIBUTIONS
Conception: D.D.S., E.J.A. and P.E.S., Writing: D.D.S., E.J.A. and P.E.S., Critical review and editing of manuscript: D.D.S., E.J.A., P.E.S., V.A.M., B.J.M., J.C., S.F.M., J.A.K., Figure design: D.D.S.
CONFLICTS OF INTEREST
NoneREFERENCE
# Corresponding Author: Daniel D. Shapiro, MD.
University of Wisconsin School of Medicine and Public Health.
Email: ddshapiro@wisc.edu.