ABSTRACT
Renal mass in a solitary kidney is a challenging clinical scenario and partial nephrectomy (PN) is generally prioritized. For tumors with high complexity, an in vivo approach with hypothermia is most often preferred at our center. Ex vivo PN is another option although results with this approach have varied and its utility in this setting is not well defined. We present the case of a 44-year-old female with bilateral renal masses including a large right renal mass with inferior vena cava (IVC) tumor thrombus and centrally located left mass with renal vein thrombus. After undergoing right radical nephrectomy/IVC tumor thrombectomy, the creatinine level was 0.96 mg/dL and she was treated with neoadjuvant axitinib to downstage the left renal mass. She then underwent laparoscopic nephrectomy, ex vivo PN on ice with excision of the renal vein thrombus, and autotransplantation into the left iliac fossa. Final pathology revealed a 7 cm pT3a grade 3 clear cell renal cell carcinoma with negative margins. During the 90- day postoperative window, she experienced acute kidney injury, a pulmonary embolism requiring systemic anticoagulation, and retroperitoneal abscess requiring drain placement, all with good recovery. At 14-month follow-up, her creatinine was 1.47 mg/dL and she has never required dialysis. She had a local recurrence at 12 months postoperatively which was successfully managed with thermoablation. In conclusion, the management of bilateral locally advanced renal tumors is complex and challenging. In rare circumstances, ex vivo PN can be considered and performed with good results.
INTRODUCTION
Renal mass in a solitary kidney is
one of the greatest challenges that
urologic oncologists can encounter.
In such cases, nephron-sparing
surgery (NSS) is prioritized to
preclude the need for dialysis. Robotic
and open partial nephrectomy
(PN) are standard options for NSS
today. However, a centrally located
mass which may not be amenable
to in situ NSS presents a unique
clinical dilemma. Such a mass may
require careful dissection of the
hilar structures, segmental vessels
and collecting system. Careful
preservation and reconstruction
of critical structures must be done
while maintaining strong oncologic
control. This is typically followed by
the need for an effective renorrhaphy
with focus on preserving as much
vascularized parenchyma as
possible. Conventional surgical
options include attempting in situ 12
open or robotic PN or performing
radical nephrectomy and placing
the patient on dialysis with potential
for later renal transplantation.
However, one technique that may be
utilized in such difficult situations
is nephrectomy followed by ex vivo
PN and autotransplantation. This
technique was utilized at Cleveland
Clinic in the early days of NSS but
was abandoned after 1986 due to
suboptimal results. We recently
reviewed our experience with renal
mass in a solitary kidney (n=1024
from 1975-2022) of which nine
patients were managed with ex
vivo PN. In this ex vivo cohort,
four patients required dialysis and
two required reoperation for renal
vein thrombus, and this approach
then fell out of favor1. Since then,
however, the introduction of fine
vessel-sealing devices, better
thrombogenic materials for packing
the renorrhaphy defect, improved
methods for capsular closure and
reconstruction, and more detailed
imaging allowing for improved
surgical planning have again made
this a potentially useful option.
Advantages of ex vivo PN
include a bloodless field which allows
for meticulous dissection of the
tumor away from hilar structures and
precise excision of minimal healthy
renal parenchyma to preserve
nephrons while obtaining negative
surgical margins. In addition, the
ability to cool and flush the kidney
with a preservative solution allows
for complex reconstruction without
strict time constraints. Small case
series and reports have shown
generally positive oncologic and
functional results for this technique
in carefully selected patients 3-7, 10.
Here we present a case of
bilateral locally advanced renal
tumors in a young morbidly obese
woman which was successfully
managed with radical nephrectomy/
IVC thrombectomy for one side and
laparoscopic nephrectomy, ex vivo
PN and renal autotransplantation
for the contralateral side, with
avoidance of dialysis.
The right renal mass was 10
cm with level 2 inferior vena cava
(IVC) tumor thrombus. The left mass
was centrally located with renal vein
thrombus and R.E.N.A.L. score
of 12. Percutaneous biopsy of the
right renal mass showed clear cell
renal cell carcinoma (RCC), grade 2.
The patient first underwent a right
radical nephrectomy and IVC tumor
thrombectomy with final pathology
showing pT3b grade 3 clear cell RCC
with negative margins. Recovery
was uneventful. Biopsy of the left
renal mass confirmed clear cell RCC,
grade 2, and she was then treated
with eight weeks of neoadjuvant
axitinib as part of a phase 2 clinical
trial to downstage the tumor in an
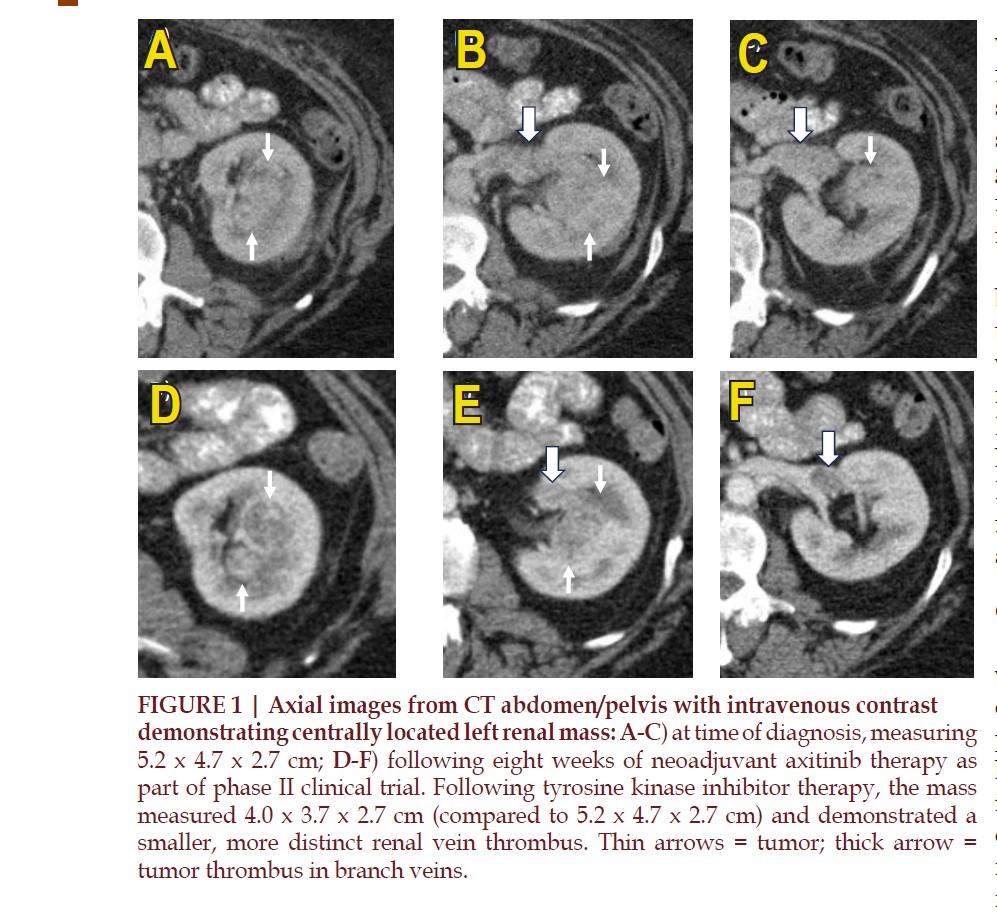
effort to facilitate PN. CT imaging
of the mass before and after
tyrosine kinase inhibitor therapy
are shown in FIGURE 1. The tumor
was downsized from 5.2 cm to 4.0
cm and the venous thrombus also
became smaller and more distinct.
After considering all options, the
decision was made to proceed with
ex vivo PN, because it was thought
that in vivo PN would be very
challenging and likely impossible
due to the tumor location, venous
involvement, and proximity to vital
structures within the hilum.
First, a left radical
nephrectomy was performed in
similar fashion to laparoscopic
donor nephrectomy using only
5 mm working ports (see video
link:
tinyurl.com/a4ps2hrv ).
Autotransplantation was then
performed with the kidney placed
into the left iliac fossa with concerns
of a possible abscess existing
Gibson incision and the vascular
anastomoses were performed to
the external iliac vessels in the
usual manner. The ureter was
reimplanted into the bladder dome
over a double-J ureteral stent.
The total operative time was 6
hours including 3 hours of back-bench
work on ice that involved resecting
the mass and reconstructing the
kidney and the renal vein. Warm
ischemia time included five minutes
during extraction of the kidney
and 25 minutes while performing
the vascular anastomoses. The
estimated blood loss was 500 cc.
Final pathology revealed a 7 cm pT3a
grade 3 clear cell RCC with negative
margins.
The patient experienced acute
kidney injury with peak creatinine
of 5.91 mg/dL on postoperative day
three which improved to 1.54 mg/
dL by six weeks with estimated GFR
of 43 mL/min/1.73 m2. She did not
require dialysis at any point during
her post- operative course. At two
months following surgery, she
was readmitted with leukocytosis
and fever and was found to have
a retroperitoneal fluid collection
concerning for possible abscess.
She underwent drain placement
by interventional radiology. Drain
culture was positive for actinomyces
neuii and was treated with a course of
antibiotics. During this stay, she was
also diagnosed with a pulmonary
embolism which was treated with
systemic anticoagulation. Both
complications were successfully
managed with conservative
measures.
At 12-month follow-up, the
patient was found to have a local
recurrence with a 3.0 cm enhancing
left anterolateral renal lesion. The
central aspect of the kidney and
the renal vein remained diseasefree.
She underwent selective
embolization and cryoablation
by interventional radiology, with
the tumor treated in two different
sittings to optimize the precision of
the treatment and minimize loss of
vascularized parenchymal volume.
Recovery was strong with a recent
serum creatinine level of 1.47 mg/dL,
correlating with an estimated GFR of
45. She is currently 16 months post-
PN and free of disease with stable
renal function. She is maintained on
close surveillance and is now also
on adjuvant immunotherapy with
pembrolizumab.
Renal autotransplantation
is an established surgical option in
highly select patients including those
with extensive ureteral stricture
disease or ureteral loss or complex
renovascular diseases extending into
the hilum. In these settings, where
the kidney itself is left undisturbed
with no loss of parenchyma and
without the need for renal capsular
closure, strong results have been
observed8,9. However, the unique
situation of ex vivo PN with
autotransplantation for renal cancer
has generally demonstrated less
favorable results, likely because of
the added complexity of combining
both procedures. Specifically, the loss
of parenchymal volume and major
renal reconstruction, combined
with the extensive ischemia that
is inherently associated with exvivo
surgery, places such patients
at high risk for acute kidney injury
and severe CKD or end-stage renal
failure on a long-term basis, and
vascular complications are also a
concern with any transplantation.
Nevertheless, nephrectomy,
ex vivo PN and autotransplantation is
still considered a potential option for
patients with renal masses with high
tumor complexity, particularly for
those with a solitary kidney. Given
the rarity of this clinical situation
and infrequent use of this technique,
the pertinent literature only includes
case reports and small series
although some of these reports have
documented encouraging oncologic
and renal functional outcomes
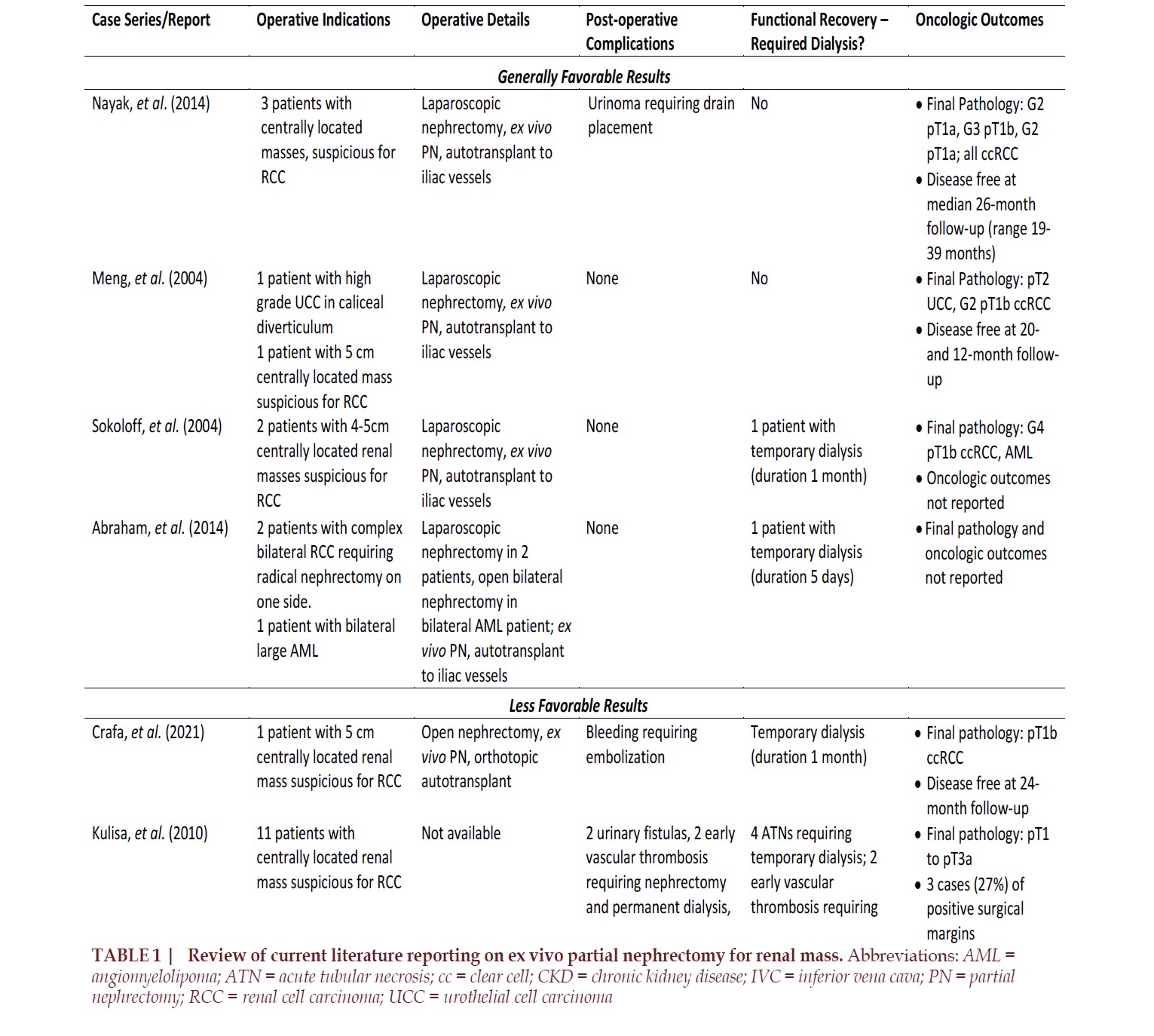
(TABLE 1)2,3,5,6. A series of three
cases of laparoscopic nephrectomy,
ex vivo PN and autotransplantation
included two patients with
functionally solitary kidneys and
one patient with stage IIIb chronic
kidney disease (CKD)3. All lesions
were pT1, clear cell RCC with negative
margins. At 19 to 39 months followup,
all patients were disease-free
with stable renal function and were
off dialysis. Another report included
a patient with high-grade urothelial
carcinoma in a calyceal diverticulum
and a patient with a 5 cm centrally
located RCC3. Both underwent
laparoscopic nephrectomy, ex
vivo PN and autotransplantation.
At 20 and 12-months follow-up,
respectively, these patients were
recurrence-free and remained off
dialysis. A series of two patients
with 4-5 cm central renal masses—
one in a patient with a solitary
kidney from previous contralateral
nephrectomy and one in a patient
with CKD—who underwent
laparoscopic nephrectomy, ex vivo PN and autotransplantation
demonstrated no short-term
postoperative complications or
dialysis requirements, although
long-term oncologic outcomes were
not reported5. Finally, Abraham et al.
reported two patients with complex
bilateral RCC and one patient with
bilateral large angiomyolipoma who
underwent ex vivo PN6. There were
no postoperative complications in
this series but one patient required
temporary dialysis. These studies
support the potential safety and
feasibility of this procedure in
appropriately selected patients.
Whether any of the above patients
might have been managed in vivo is
of course very difficult to assess.
Other series demonstrate the
possible complications associated
with this complex surgery (TABLE
1)4,7,10. A case report of a 77-yearold
female with a 5 cm hilar mass
who underwent open nephrectomy
through a midline incision followed
by ex vivo PN and orthotopic
autotransplantation highlights some
difficulties that can be encountered.
Following ex vivo PN in this case, the
kidney was returned to its orthotopic
position and end-to-end arterial and
venous anastomoses were performed
as well as a uretero-ureterostomy
over a double-J ureteral stent. The
patient required hemodialysis for
one month postoperatively and
developed bleeding from a lower
pole pseudoaneurysm requiring
embolization on postoperative day
10. The patient was disease-free with
stable renal function at 24-month
follow-up4. Furthermore, a series of
11 patients who underwent ex vivo PN
for complex renal tumors from 1996-
2009 showed a complication rate
of 73% which included two urinary
fistulas, two vascular thromboses
requiring nephrectomy and dialysis,
two pulmonary complications and
four patients required temporary
dialysis7. Tumor stage ranged from
pT1 to pT3a and there were three
cases of positive surgical margins
(27%). In this series there were two
local recurrences (18%) and five
cases of progression to metastatic
disease (45%) leading to two deaths
(18%).
The largest case series on ex
vivo PN published to date included
five patients treated for complex
RCC, five for complex upper tract
urothelial carcinoma, one for
isolated metastasis to the kidney,
and one for renal nephroblastoma11.
Postoperatively, two patients
required temporary dialysis. Median
follow-up was 84 months, and six
patients (50%) eventually died with
functioning grafts. There were five
cases of local or distant recurrence
and two additional patients died
from their disease. Only one person
developed end stage renal disease
requiring dialysis. These studies
highlight that strict patient selection
is key and that oncologic safety
should not be compromised during
ex vivo PN. At our institution, ex
vivo PN has only been considered
primarily for patients with RCC, in
contrast to some studies highlighted
above with broader selection criteria.
Our patient described in this study
had pT3a disease with negative
margins, although a local recurrence
was identified that required salvage
therapy, specifically thermoablation.
The initial suboptimal results at
our center with ex vivo PN led to
abandonment of this approach for
36 years, until the present case. We
recently reviewed all 1024 cases
of renal mass in a solitary kidney
from 1975-2022 at Cleveland Clinic
of which 10 cases were managed
with ex vivo PN, including the case
presented here and nine other cases
prior to 19861. Unfortunately, in our
previous nine cases, four required
dialysis and two required reoperation
for renal vein thrombosis and this
approach fell out of favor due to
these suboptimal results.
The technique described
here of laparoscopic nephrectomy,
ex vivo PN and autotransplantation
is advantageous due to its ability
to preserve maximal vascularized
parenchymal volume and preclude
the need for dialysis which has its
own well- established morbidity.
Good oncologic control is critically
important and with the kidney on
ice, frozen sections can be obtained
and additional resection can be
performed if necessary in an effort to
achieve an R0 result. Most reports—
including our own—utilized
laparoscopic nephrectomy rather
than open which may further reduce
the morbidity of the approach.
Disadvantages include longer
operative time as this technique is
essentially the combination of three
urologic procedures. In addition
to known complications of partial
nephrectomy including urine leak
and postoperative bleeding from
the reconstructed kidney, are also
the risks of renal transplant such
as bleeding from the vascular
anastomoses, acute arterial or
venous thrombosis, arterial stenosis
or ureteral stricture. This procedure
also requires a surgeon or team
of surgeons experienced in both
nephron-sparing surgery and renal
transplant. It is important to keep
in mind that if good oncologic
control cannot be achieved, then the
procedure should be converted to
radical nephrectomy and the patient
can potentially be listed for delayed
renal transplantation.
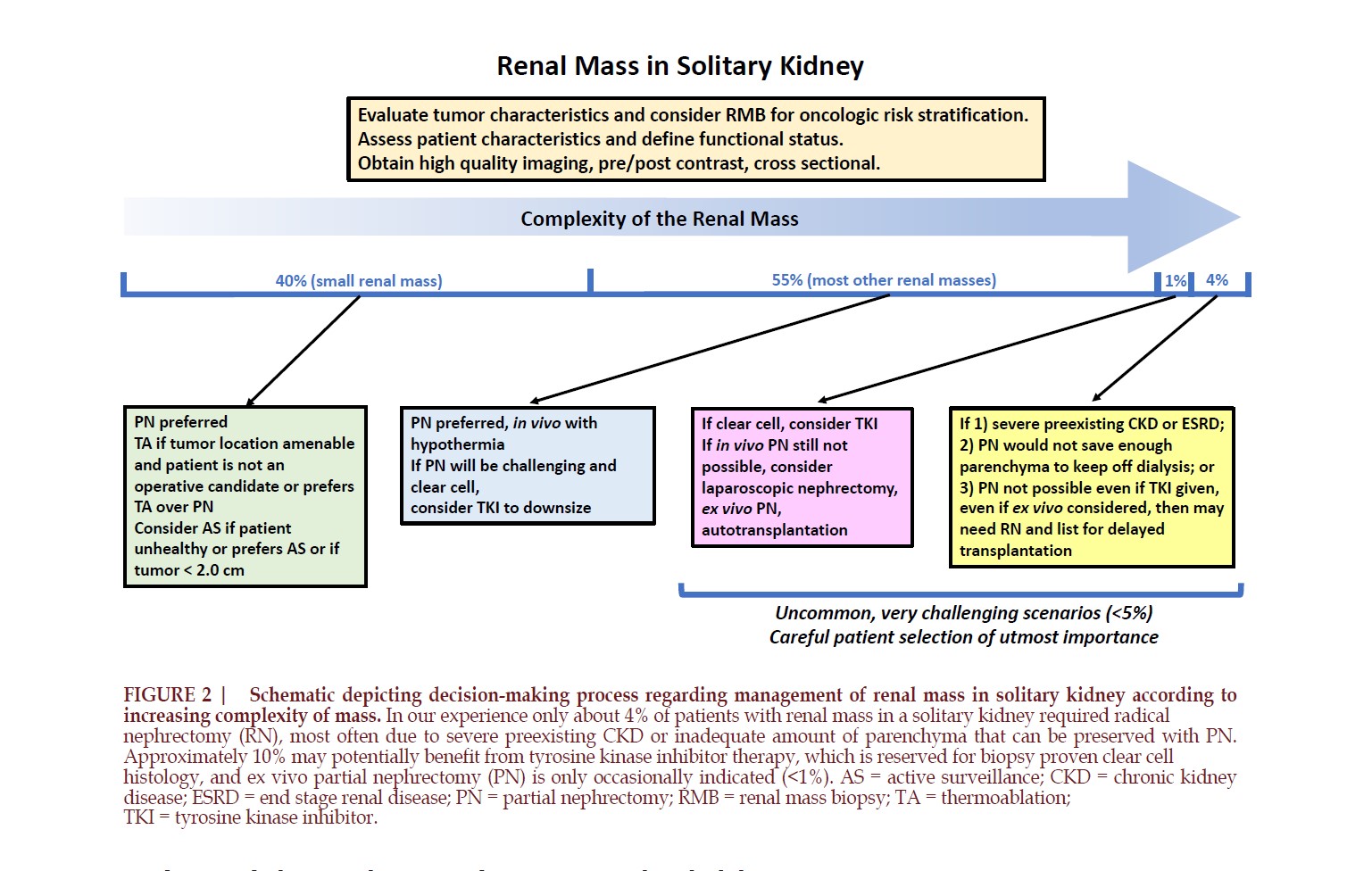
In conclusion, laparoscopic
nephrectomy, ex vivo PN and
reconstruction with hypothermia,
and autotransplantation is a viable
approach for managing the most
complex of renal masses in a
solitary kidney (FIGURE 2). With
improved technology, including
fine vessel-sealing devices, better
thrombogenic materials for packing
the renorrhaphy, improved methods
for capsular closure, and more
detailed imaging allowing for more
intelligent surgical planning, this
has become a more feasible option
which we are now adding back to our
armamentarium. However, careful
patient selection is of paramount
importance, as this procedure
naturally comes with increased
perioperative risks, and should
only be used when essential. For
the great majority of complex PN
in a solitary kidney, our preference
remains in favor if in vivo PN with
hypothermia. The morbidity that can
be associated with ex vivo PN and
autotransplantation likely reflects
the complexity of the cases, and good
judgment is required to determine
where the threshold should be for
abandonment of PN with conversion
to RN for such patients.
FUNDING: This research did
not receive any specific grant from
any funding agency in the public,
commercial or not-for-profit sector.
Conflicts of Interest: None
CASE PRESENTATION
A 44-year-old female
was found to have bilateral solid,
enhancing renal masses on imaging
performed during work-up for
hypertension. Her comorbidities
included hypertension and morbid
obesity (BMI 50). She reported
right flank discomfort. There was
no family history of genitourinary
malignancy or other findings
that would suggest familial RCC.
Metastatic work-up was negative.
Her baseline serum creatinine level
was 0.89 mg/dL.
An
additional 10 mm port was placed
in the left lower quadrant and
this was utilized for the vascular
stapler and specimen bag. This
port site was extended in Gibson
fashion for specimen extraction.
The kidney was then chilled on ice
and perfused with hypothermic
Collins' solution before being
defatted and undergoing ligation of
small vascular branches. The central
mass with extension into the vein
was visualized on ultrasound and a n
anterior wedge of parenchyma was
excised to allow for hilar and tumor
ex posure. The renal vein was opened
and the tumor thrombus resected
followed by sequentia l dissection
of tumor out of the central aspec t of
the kidney. A fine vessel sealer was
used when necessary to ligate small
vessels feeding the tumor. Af ter
oversewing the parenchyma, suturing
rema ining transected vessels, and
packing the defect with FibrillarTM
(Ethic on, Cincinnati, OH), the outer
renorrhaphy was performe d with
2- 0 chromic sutures as shown in the
surgical video above.
DISCUSSION
We present a very challenging
case of a 44-year-old female with
morbid obesity and hypertension
found to have bilateral renal lesions
with venous involvement who
underwent right radical nephrectomy
with IVC thrombectomy followed by
left laparoscopic nephrectomy, ex
vivo PN and autotransplantation.
At 16-months follow-up, she has
good renal function with creatinine
of 1.5 mg/dL and has not required
dialysis. She had local recurrence
that was managed with selective
embolization and thermoablation
and is now disease-free on adjuvant
immunotherapy.
REFERENCE
# Corresponding Author: Steven C. Campbell, MD, PhD.
Department of Urology, Glickman Urological and Kidney Institute,
Cleveland Clinic Cleveland, OH, 44195. Campbes3@ccf.org