Submitted - October 18, 2022 | Revised December 4, 2022 Accepted - December 7, 2022 | | ePublished - December 31, 2022
https://doi.org/10.52733/KCJ21n1-r1
ABSTRACT
Renal Cell Carcinoma (RCC) is among the most frequently diag-nosed cancers in the United States. One-third of patients present with metastatic disease, and up to another half may progress to metastasis following surgical treatment. Survival rates for metastatic RCC have risen over the past 20 years, an improvement partially attri-butable to the increased availability of immune checkpoint inhibitors (ICI). However, mRCC remains a fatal genitourinary cancer, with pa-tients often demonstrating both primary and secondary resistance to available immunotherapies. Sarcopenia, inflammation and nutrition have emerged as important prognostic factors in RCC. Recent studies have demonstrated their impact in predicting efficacy and tolerability of ICIs for RCC and other advanced solid malignancies. In this review, we aim to highlight the major milestones in ICI therapy for RCC, and associated mechanisms of action. We also examine how sarcopenia, inflammation and nutrition affect outcomes in RCC, particularly with consideration of the impact on immunotherapy efficacy and toxicity.
INTRODUCTION
Renal Cell Carcinoma (RCC) is
among the top 10 cancer diagnoses in
the United States, with an estimated
79,000 new cases and 14,000 deaths
in 2022.1
The incidence has doubled
over the past half-century, likely
attributed to improved and more
frequent imaging.2 Nevertheless,
one-third of patients present with
distant metastatic disease and 20-
50% progress to metastasis despite
surgical resection.3 Over the past
decade, the 5-year survival rate for
metastatic RCC (mRCC) has risen
from 12% to 15.3%,1,3 an improvement
at least partially attributed to the
increased availability of systemic
treatment options. Primary systemic
therapy options for RCC include
vascular endothelial growth factor
(VEGF)-targeted tyrosine kinase
inhibitors (TKI) and the more recent
introduction of immune checkpoint
inhibitors (ICI) such as nivolumab,
ipilimumab, pembrolizumab and
avelumab. The development of
immune checkpoint blockade with
antibodies against programmed cell
death protein 1 (PD-1), programmed
cell death ligand 1 (PD-L1), and
cytotoxic T-lymphocyte-associated
antigen 4 (CTLA-4) has resulted in
significant and durable responses
in RCC with acceptable safety.4–10
Multiple phase III randomized
clinical trials comparing ICI
monotherapy and combination
therapies against targeted therapies
for RCC have demonstrated higher
median overall survival (OS) and
progression-free survival (PFS)
with improved objective response
rates (ORR).4–8,11 This has resulted
in a major shift towards ICI-based
combination therapies as preferred,
first-line options for the management
of advanced RCC.12
However, ICI efficacy and tolerance may be impacted by other factors, such as sarcopenia, inflammation, and nutritional status, which influence survival outcomes in patients with cancer. Sarcopenia is a progressive and generalized skeletal muscle disorder with accelerated loss of muscle mass and function associated with increased risk of falls, frailty, and mortality.13 Although observed in the context of aging, sarcopenia additionally occurs concurrently or independently in the setting of cancer,14,15 where there is malignancy-related weight loss and muscle wasting known as cancer cachexia.16 Sarcopenia and its association with worse survival has been widely reported in patients with RCC, especially in patients with advanced or metastatic disease.14,17–24 Similarly, markers of malnutrition and inflammation, such as C-reactive protein (CRP), low body mass index (BMI), hypoalbuminemia and neutrophil, lymphocyte, and platelet counts, have also been associated with survival in RCC and other malignancies.25–29
In addition to influencing survival in RCC, studies have documented the impact of these factors on the efficacy and tolerability of ICI treatment. Here, we briefly review the major milestones in ICI therapy for advanced RCC and associated mechanisms of action. We focused on data from clear cell RCC as the most commonly encountered histology, recognizing that much of our management of non-clear cell subtypes are extrapolated from this body of work. Then, we examine sarcopenia, inflammation, and malnutrition in RCC and consider its impact on immunotherapy efficacy and tolerance and discuss future considerations for guiding management.
IMMUNE CHECKPOINT INHIBITORS IN ADVANCED RENAL CELL CARCINOMA
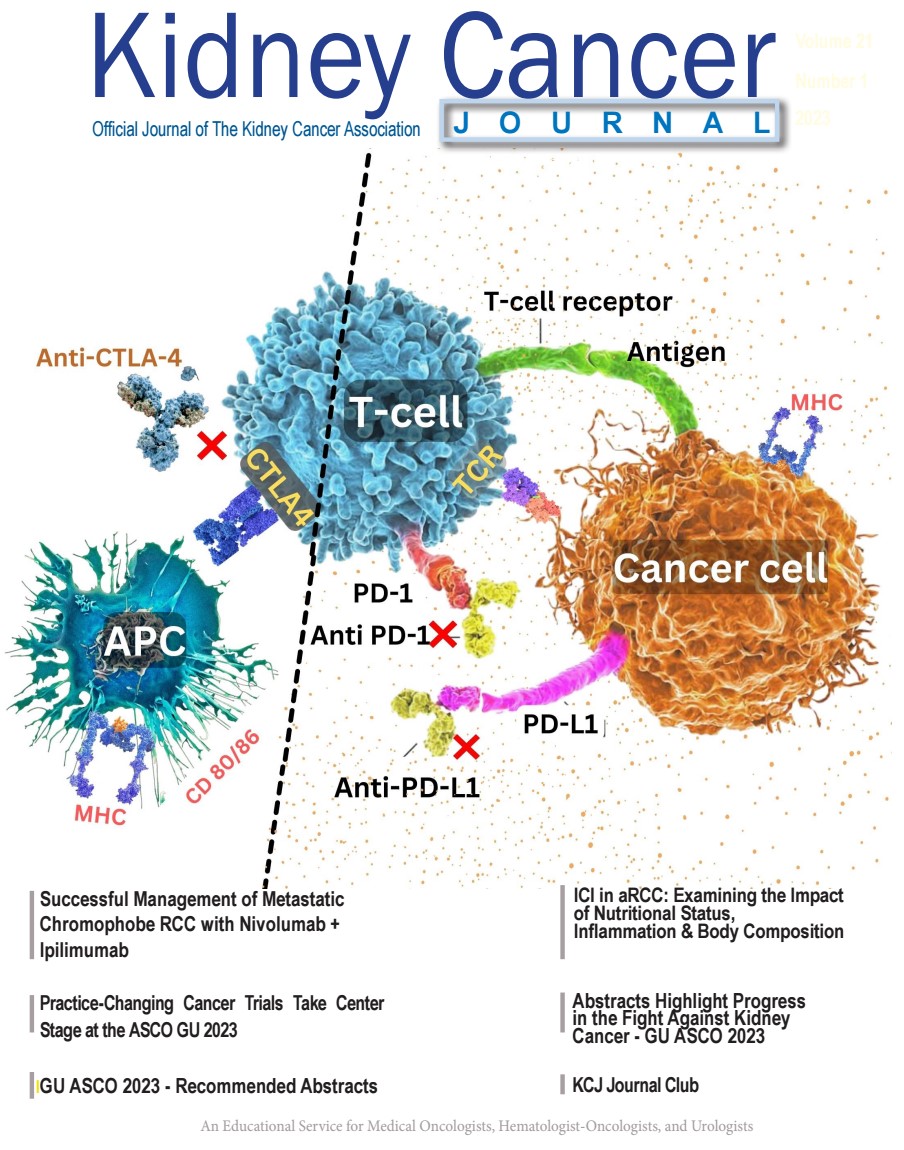
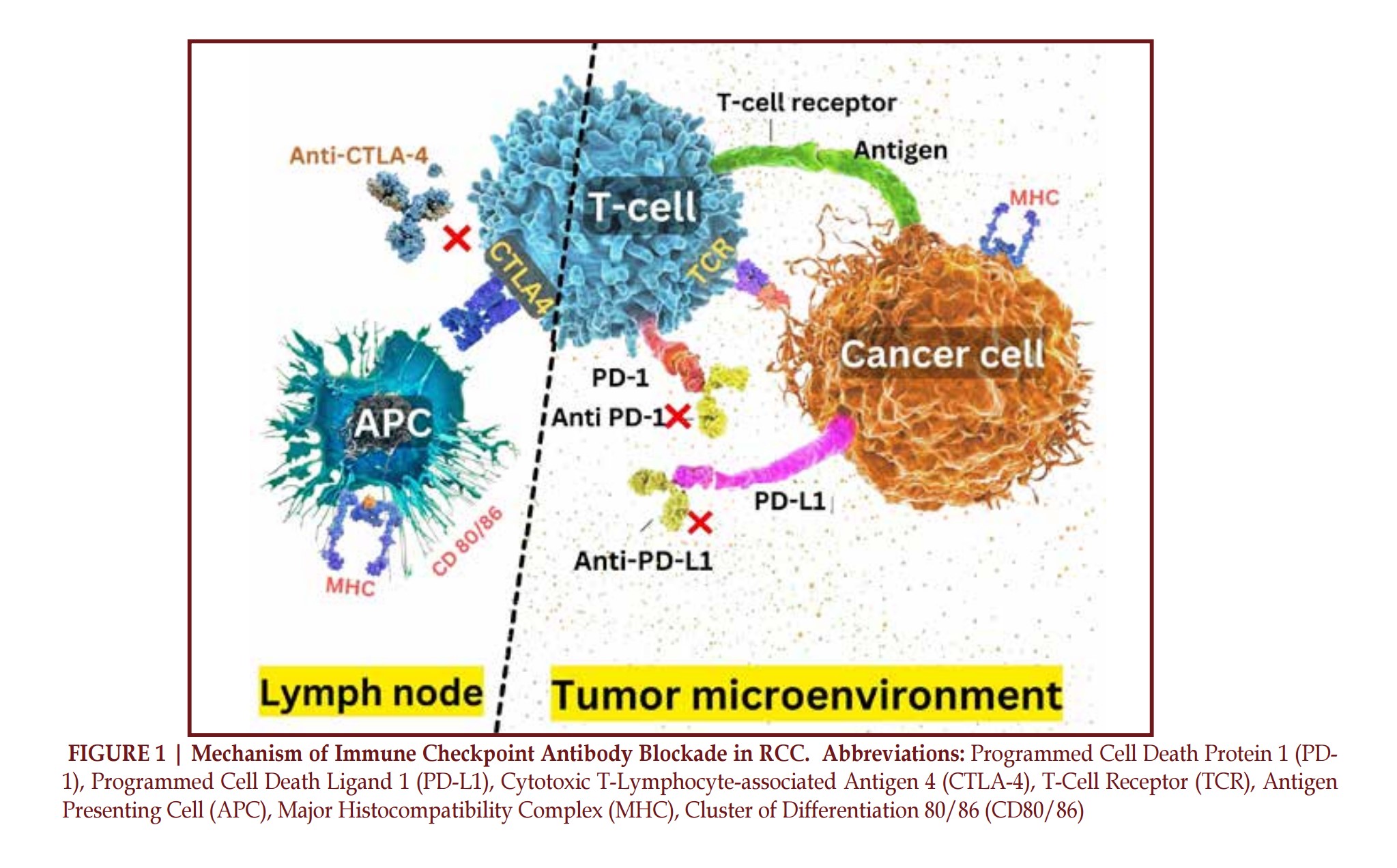
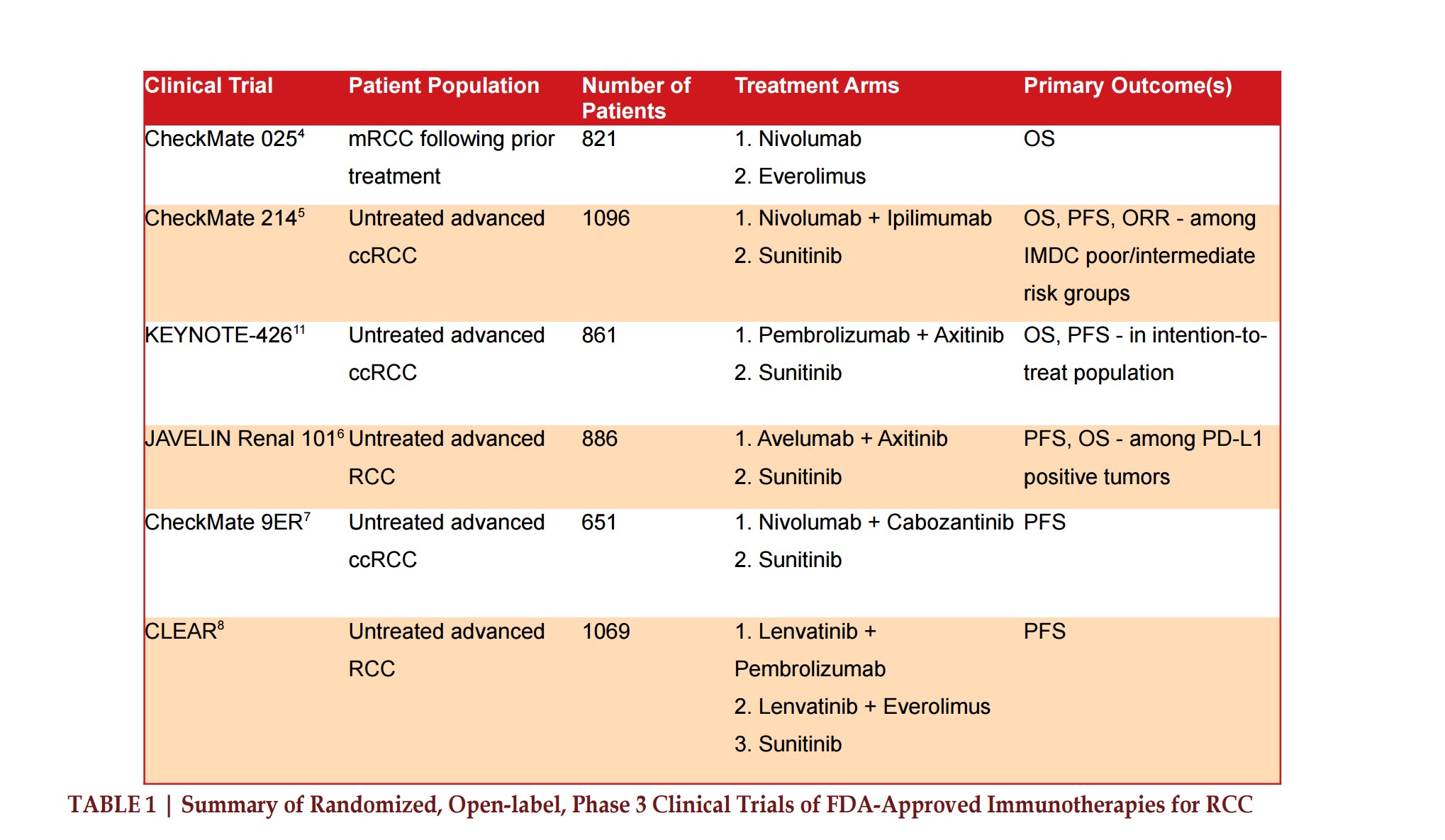
Numerous immunotherapies have been studied and received approval for treatment of RCC since 2015. A representative summary of these randomized controlled trials are summarized in Table 1. A summary of the mechanism of immune checkpoint inhibition is also represented in Figure 1.
History of Immune Checkpoint Inhibitors
The FDA approved the first ICI, ipilimumab (CTLA-4 checkpoint inhibitor), in 2011 for metastatic melanoma.30,31Then, in 2014, the FDA approved the first PD-1 checkpoint inhibitor, nivolumab.30,31 The phase 3 CheckMate 025 trial, published in 2015, compared nivolumab versus everolimus in mRCC following prior treatment, which demonstrated longer median OS (25.0 months [95% confidence interval, 21.8 to not estimable] vs 19.6 months [95% CI, 17.6-23.1]) with less grade 3-4 treatment related adverse events (TRAE), but no difference in progression free survival (PFS, 4.6 [95% CI, 3.7-5.4] vs 4.4 months [95% CI, 3.7-5.5]).4 Nivolumab for the treatment of mRCC after treatment with standard antiangiogenic therapy was then approved. Combination therapy of nivolumab plus ipilimumab versus sunitinib in previously untreated mRCC was studied in the phase III Checkmate 214 trial. This showed significantly longer OS (median OS not reached [95% CI, 28.2 months to not estimable] versus 26.0 months [95% CI, 22.1 to not estimable]), higher objective response rate (ORR, 42% [95% CI, 37-47] vs 27% [95% CI, 22-31], p<0.0001) and complete response rate (CRR, 9% vs 1%), which led to FDA approval as first-line treatment for intermediate to poor-risk advanced RCC in April 2018.5,31 In the long-term analysis with minimum 42-month follow-up, duration of response was longer, and more patients achieved complete response with nivolumab plus ipilimumab regardless of International mRCC Database Consortium (IMDC) risk group.32
Pembrolizumab, another PD-1
checkpoint inhibitor, was first
approved in 2014 for advanced
melanoma, and showed antitumor
activity in untreated mRCC.33 The
KEYNOTE-426 trial comparing
pembrolizumab plus axitinib, an
anti-VEGF TKI, versus sunitinib for
treatment-naive advanced ccRCC
showed a 12-month OS benefit
(89.9% [95% CI, 86.4-92.4] vs
78.3% [95% CI, 73.8-82.1]) with a
longer PFS (15.1 [95% CI, 12.6-17.7]
vs 11.1 months [95% CI, 8.7-12.5])
and improved ORR (59.3% [95%
CI, 54.5-63.9] vs 35.7% [95% CI,
31.1-40.4], p<0.001). These results
were observed across all IMDC
risk groups regardless of PD-L1
expression.11 FDA approval followed
soon after in April 2019 as first-line
combination immunotherapy for all-risk advanced RCC.
The first PD-L1 checkpoint inhibitor that received approval for mRCC was avelumab with combination axitinib in May 2019. This was supported by the phase III JAVELIN Renal 101 trial of avelumab plus axitinib as compared with sunitinib in patients with previously untreated advanced RCC. Primary endpoints focused on PFS and OS among patients with PD-L1 positive tumors. The median PFS among this cohort was significantly longer for patients that received avelumab plus axitinib (13.8 [95% CI, 11.1 to not estimable] vs 7.2 months [95% CI, 5.7-9.7]), and in the overall population, PFS was also longer (13.8 [95% CI, 11.1 to non estimable] vs 8.4 months [95% CI, 6.9-11.1]).6
In 2021, the FDA granted approval to the two remaining frontline combination immunotherapies for advanced RCC treatment: cabozantinib (TKI) plus nivolumab, and lenvatinib (TKI) plus pembrolizumab. The phase III CheckMate 9ER trial comparing nivolumab plus cabozantinib versus sunitinib for advanced RCC showed benefits in median PFS (16.6 [95% CI, 12.5-24.9] vs 8.3 months [95% CI, 7.0-9.7]) and ORR (55.7% [95% CI, 50.1-61.2] vs 27.1% [95% CI, 22.4- 32.3], p<0.001). Grade 3 or higher TRAEs were similar, with patients also reporting better health-related quality of life with the combination regiment, demonstrating its acceptable safety profile.7 In the CLEAR trial comparing lenvatinib plus pembrolizumab or everolimus versus sunitinib for advanced RCC, significant benefits were observed with the immunotherapy-containing regimen in terms of PFS (23.9 [95% CI, 20.8-27.7] vs 9.2 months [95% CI 6.0-11.0]), OS at 24 months (79.2% vs 70.4%; hazard ratio [HR] for death, 0.66 [95% CI, 0.49-0.88]; p=0.005), and ORR (71.0% vs 36.1%; relative risk [RR], 1.97 [95% CI, 1.69-2.29]) versus sunitinib.
These immunotherapy regimens represent the approved, first-line and preferred options for the treatment of RCC, with many other immune-checkpoint inhibitor-based combinations or monotherapies currently under investigation or awaiting approval12,34–38.
Interplay between ICIs and RCC
The tumorigenesis and development of RCC is well documented. Clear cell RCC frequently contains multiple loss-of-function mutations in the tumor suppressor gene Von Hippel-Lindau (VHL). This results in the induction of hypoxia inducible factors (HIF), which promotes cells to express VEGF and other factors that increase tumor angiogenesis and growth.39 These findings were the basis for anti-angiogenic agents becoming the standard of care for advanced RCC. These drugs demonstrated improvements in OS and PFS, but without significant complete or durable response rates as monotherapies.40
It has become better documented how multiple subtypes of RCC share alterations of specific pathways involving metabolism, hypoxia, and immune checkpoints.41, 42 RCC is notably associated with a highly inflammatory microenvironment with increased frequency of tumor infiltrating lymphocytes.43 Despite prominent levels of T-cells within tumors, RCC often escapes via immunosuppressive mediators from the microenvironment or tumor cell overexpression of CTLA-4 and PD-L1 which block T-cell responses.43 This infiltrate is partially composed of regulatory T cells (Treg), which can prevent cancer antigen recognition, and reduce the antitumor activity of lymphocytes present.44 Markers associated with T-cell exhaustion along with the promotion of Th2 induction have been identified, which can allow for unchecked tumor growth in a state of chronic inflammation.41, 45 These findings support the use and improved benefits associated with immunotherapy in the treatment of RCC. However, many patients may not respond to immunotherapy and durable responses remain an exception, which can reflect the presence of primary and secondary resistance to ICIs.
There are multiple theories that explain resistance including certain patient-intrinsic, tumor cell-intrinsic, and tumor microenvironment factors.46 One explanation is the tumor cell-induced release of VEGF which promotes abnormal neovascularization, Treg proliferation, and reduces CD8+ T-cell proliferation and penetration into the tumor. This supports the rationale for combining ICIs and anti-VEGFR TKIs as dual therapy for mRCC to target both antitumor processes.40, 47Other explanations for potential ICI resistance include Wnt/ß-catenin pathway overexpression leading to T-cell exclusion and resistance to anti-PD(L1) and CTLA-4 antibodies along with MAP Kinase alterations that inhibit T-cell recruitment and function.46 For patients that do respond to ICIs there is often a robust activation of CD8+ T-cells within the microenvironment, along with increased interferon-gamma signaling that promotes acute inflammation.48 However, over time, evidence suggests an adaptation to increased T-cell checkpoint molecule expression that can lead to immunotherapy resistance.48 Patient-specific factors, including sarcopenia, systemic inflammation and markers of nutritional status, remain an important barrier to immunotherapy efficacy and can be identified and addressed for improved management of advanced RCC.
SARCOPENIA, INFLAMMATION, AND MALNUTRITION IN ADVANCED RENAL CELL CARCINOMA
Definitions, Epidemiology, Relationships, and Pathophysiology
Sarcopenia is a generalized skeletal muscle disorder defined by 3 main criteria: low levels of muscle strength, muscle quantity and/ or quality, and decreased physical performance which can indicate severity.13,49 Cross-sectional imaging with computed tomography (CT) or magnetic resonance imaging (MRI) is widely prevalent during RCC screening, staging, and follow-up and can additionally be used to evaluate for sarcopenia at the third lumbar vertebra (L3), which correlates well with total skeletal muscle mass.50-53 Commonly, the skeletal muscle index (SMI, cm2/m2) is calculated by dividing cross-sectional area of skeletal muscle at L3 by the patient’s height in meters squared.54 Then, SMI thresholds are used to define sarcopenia vs. nonsarcopenia; however, it should be noted that there is wide variation in SMI thresholds used to define sarcopenia, which is an important consideration for future incorporation and study interpretation.55There has been further investigation since sarcopenia was first defined to clarify specific categories including primary and secondary forms, acute and chronic sarcopenia, sarcopenic obesity, and malnutrition-associated sarcopenia.49 Primary sarcopenia refers to age-related changes, where, in addition to hormonal, physical activity, and nutritional changes, a state of chronic low-grade inflammation can contribute to the loss of muscle over time.49,56 Based on established thresholds for muscle mass, up to 20% of those aged 70-79 and 30% of the population 80 or older meets this criterion for sarcopenia.57 In addition, studies have demonstrated a high prevalence of weak muscle strength and decreased physical performance in populations aged 65 or older, affecting up to half of all individuals.57
Normal aging is associated with elevated levels of pro-inflammatory markers, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and C-reactive protein (CRP), often associated with long-standing mitochondrial and immune dysfunction, cellular injury, and increased adiposity.58 Multiple studies have demonstrated that higher levels of circulating cytokines, including TNF-α and IL-6, are associated with loss of skeletal muscle mass and strength, with an overall increased risk of sarcopenia.59–61 In a separate meta-analysis, CRP is suggested to be a potential parameter for detecting sarcopenia given its association with higher serum levels in sarcopenic patients.62 Alterations in pro-inflammatory markers can, directly and indirectly, affect skeletal muscle metabolism by increasing catabolic pathways for muscle breakdown, and preventing appropriate use of proteins for muscle synthesis.56
Systemic inflammation is also associated with solid malignancies and can exacerbate typical age-related skeletal muscle mass loss and contribute to worse outcomes. In a meta-analysis of over 80,000 patients with malignant tumors, sarcopenia was identified in 35.3%, and varied between 35-50% in RCC.15 Cancer and its treatments can increase the risk of developing sarcopenia via the promotion of anorexia, physical inactivity, and pro-inflammatory states, along with treatment related damage to muscle tissue.63 The development of sarcopenia can also co-occur as a component of cancer cachexia, defined as a progressive, multifactorial syndrome with continuous loss of skeletal muscle mass resulting in functional impairment that cannot be fully reversed.16 Cancer cachexia arises from a combination of systemic inflammation and negative energy balance and affects ~30% of all cancer patients and close to 80% of patients with metastatic disease to the brain.64 The diagnosis requires certain changes in overall weight, BMI, and sarcopenic criteria.16 Furthermore, advanced cancer patients are often affected by nutritional impact symptoms, including anorexia, nausea, vomiting, taste, and smell changes, as a result of chemotherapy, radiotherapy, and even systemic inflammation that can alter hunger/ satiety signaling thus preventing compensation for the ongoing negative energy balance.64
General Impact of Sarcopenia, Inflammation and Malnutrition on Survival in RCC
Sarcopenia is associated with poor OS and CSS across a wide variety of non-hematological solid tumors.65 In a systematic review examining treatment-related outcomes for patients undergoing nephrectomy for localized and mRCC, sarcopenia was an independent predictor of mortality, especially following systemic treatment.66 In patients with non-mRCC treated with radical nephrectomy, Psutka et al found sarcopenia as inferior 5-year CSS (79% vs 85%, p=0.05) as well as inferior 5-year OS (65% vs 74%, p=0.005).19 In a study of mRCC patients, sarcopenia was associated with a 2.5x higher risk of all-cause mortality. and improved the prognostic ability of the MSKCC risk model when included with or substituted for Karnofsky performance status.21 Similar results have been found in other cohorts of patients with metastatic and nonmetastatic RCC.18, 67
Increasingly, sarcopenia with other markers of inflammation and nutrition are being considered and have demonstrated an association with increased mortality.17,18,20,68 Higher modified Glasgow prognostic scores (mGPS), which features CRP and albumin as measures of inflammation and nutrition, have been associated with worse OS, CSS, RFS, and PFS, and have an even greater association when combined with sarcopenia.18,29,69 Other studies have analyzed the predictive impact of the prognostic nutritional index (PNI) in patients undergoing nephrectomy, as calculated by albumin and lymphocyte levels.26 Increases in PNI scores have shown a decreased risk of death from RCC.25 PNI also demonstrated greater prognostic ability for both OS and PFS when compared to other inflammatory measures, such as Neutrophil-to-Lymphocyte (NLR), Platelet-to-Lymphocyte (PLR), and Lymphocyte-to-Monocyte (LMR) ratios.25,26 On univariate analysis, these indices were associated with shorter OS and PFS, but only PNI was significant on multivariable analysis.26 Multiple methods of evaluating for sarcopenia, inflammation, and nutritional status exist and demonstrate prognostic utility in localized and advanced RCC.
IMPACT OF SARCOPENIA, MALNUTRITION, AND INFLAMMATION ON IMMUNE CHECKPOINT EFFICACY
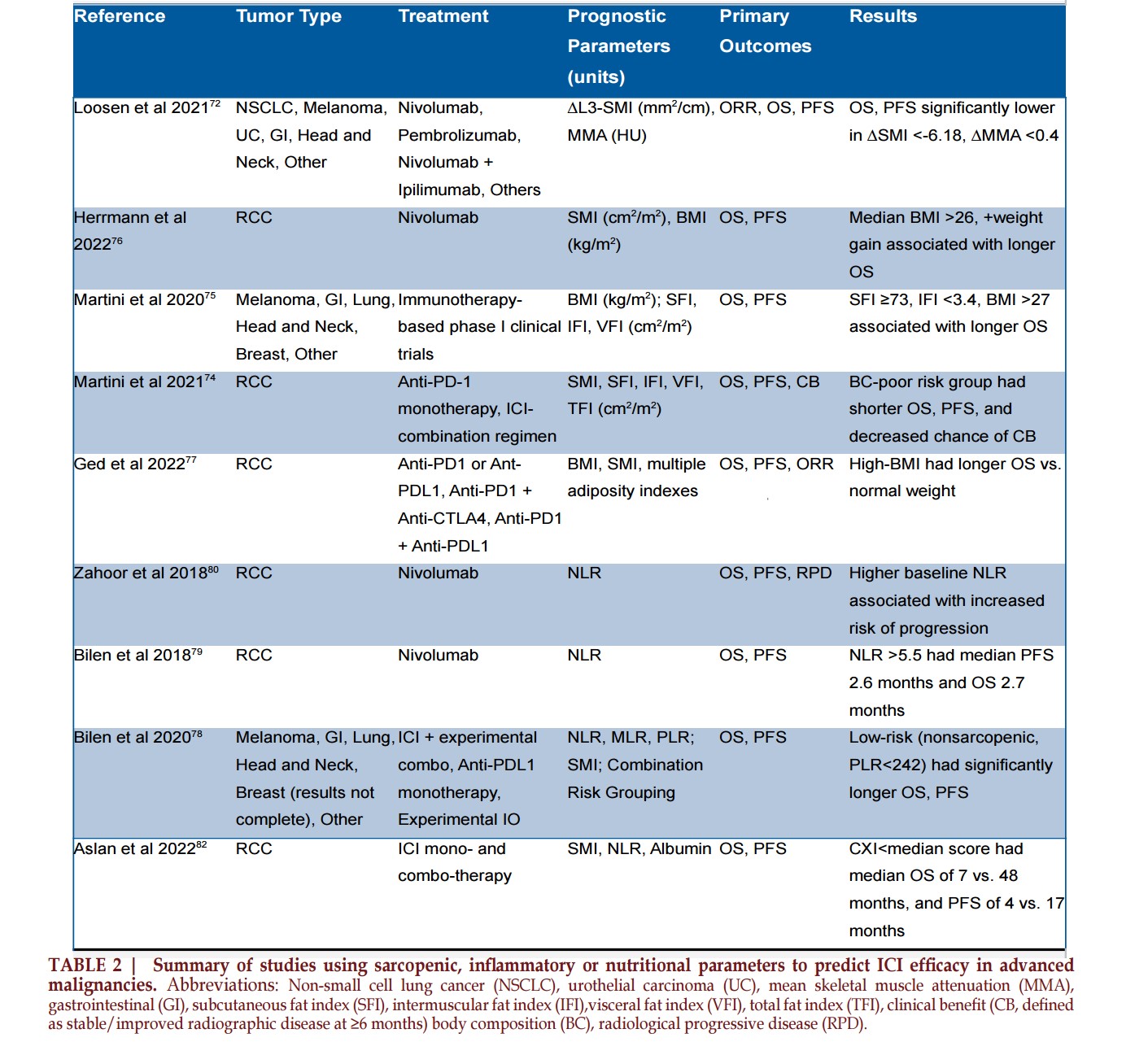
Examination of ICI efficacy and toxicity in relation to sarcopenia and other markers of nutrition and inflammation has emerged over the past decade. A representative summary of studies examining these interactions is summarized in Table 2.
Sarcopenia
A retrospective analysis of patients with advanced cancer receiving ICIs found sarcopenic patients experienced worse ORR (15.9% vs 30.5%, p=0.095) although this was statistically insignificant.70 However, 1-year PFS (10.8% vs 32%; RR, 1.31; p<0.001) and OS (43% vs 66%; RR 1.71; p<0.001) were significantly lower for the sarcopenic patients.70 In another group of patients with advanced solid tumors that received ICI monotherapy, sarcopenia prevalence was nearly 50% and a significant predictor of worse OS, PFS, and ORR and not dependent on the type of ICI received.71In addition to baseline muscle measurements, longitudinal change during ICI therapy has additionally exhibited prognostic ability. In one prospective study, 88 patients received either nivolumab (55.7%), pembrolizumab (28.4%), or nivolumab plus ipilimumab (9.1%) for various solid organ malignancies.72Although no difference in baseline SMI between responders vs. non-responders was observed, patients that responded to ICI therapy at the 3-month mark experienced an increase in SMI (+1.73 vs -3.20 mm2/cm, p=0.002) and median muscle attenuation (+0.89 vs -1.0 HU, p=0.090), an indicator of muscular fat deposition.72 Furthermore, OS was significantly lower (127 vs 547 days, p<0.001) in patients that experienced a strong decline in SMI (<-6.18 mm2/ cm) or muscle attenuation (<-0.4 HU) compared to patients with stable or mild decreases.72 The progressive loss of muscle mass with increased myosteatosis might reflect increased malignancy-associated inflammation which may negatively influence the antitumor effects of ICIs.73
Alternative Body Composition Parameters
In addition to quantified muscle composition, other parameters such as BMI, adipose distribution, and muscle quality may be informative. In an analysis of 79 patients treated with ICI for mRCC, Martini et al measured density (as measured via HU) of skeletal muscle, subcutaneous fat, intramuscular fat, and visceral fat in addition to SMI. Patients were stratified into poor, intermediate, or favorable risk groups based on these measurements, with the poor risk groups experiencing significantly shorter OS, PFS, and lower chance of radiographic response at 6 months compared to the favorable risk group.74 Furthermore, a lower total fat index was also associated with shorter OS, PFS, and a lower chance of radiographic response.74 These findings suggest that, in addition to muscle quantification, markers of adiposity and muscle quality (i.e. intramuscular fat) may be informative and predict outcomes for patients with RCC receiving ICI therapy. This aligns with prior studies demonstrating that increased BMI, weight gain, increased subcutaneous fat index, and decreased intermuscular fat index during ICI treatment are associated with prolonged survival or treatment response in patients with cancer,75 including mRCC.76,77
Inflammation
Relationships between inflammation and body composition in patients receiving ICI have also been considered. In 90 patients enrolled in immunotherapy-based phase 1 clinical trials, Bilen et al. risk-stratified patients based on sarcopenia measurements and baseline inflammatory markers (i.e. NLR, MLR, and PLR). A negative correlation was observed between SMI and PLR, and very high-risk (PLR ≥242 and sarcopenic) or intermediate (PLR <242 and sarcopenic) risk groups experienced significantly shorter OS and PFS compared with low-risk patients (PLR <242 and non-sarcopenic).78 In a separate study of 38 mRCC patients treated with nivolumab, Bilen et al demonstrated that low NLR values were associated with longer median PFS (not estimable vs 2.6 months; HR 0.20 [95% CI, 0.07-0.64; p=0.006]) and OS (not estimable vs 2.7 months; HR 0.06 [95% CI, 0.01-0.55; p=0.012]).79 These findings were echoed by Zahoor et al, where a higher baseline NLR was associated with an increased risk of progression in mRCC patients treated with nivolumab.80 It is well documented how both inflammation and sarcopenia contribute to worse outcomes in malignancy and can limit treatment efficacy, but the inclusion of multiple markers for risk stratification may better account for multiple underlying prognostic factors.
Nutritional Status
Advanced RCC patients are often susceptible to malnutrition and resulting cancer cachexia, which can affect ICI efficacy. As previously discussed, higher PNI is associated with better survival. In a series of studies from Asian countries looking at PNI and survival outcomes in advanced cancer patients treated with ICIs, higher PNI was associated with greater ORR and longer OS and PFS.81 The cachexia index is another combined score of sarcopenic and inflammatory markers used as a prognostic model in cancer patients. This index, based on SMI, NLR, and albumin levels, was used in a retrospective review of 52 mRCC patients who had received ICI as a 2nd-line or later treatment.82 Below median cachexia index score was found to significantly affect OS (7 vs 48 months; HR 4.5 [95% CI, 1.9- 11; p=0.001]) and PFS (4 months vs. 17 months; HR 2.6 [95% CI, 1.3-5.3; p=0.007]) as opposed to the other markers.82 One theory for why the procatabolic and proinflammatory state associated with cancer cachexia may interfere with ICI efficacy is increased clearance and metabolism. A prospective cohort study on the pharmacokinetics of nivolumab used in advanced cancers, including 14 patients (6.3%) with mRCC, showed how increased body-surface area and decreased albumin were associated with increased clearance of the ICI.83 A clearance-response trend was observed in mRCC where clearance was higher in patients with progressive disease, although this was non-significant.83 However, this trend was significant in NSCLC (n=158; 71.5%), and given the smaller percentage of patients with mRCC, the study may have been underpowered to demonstrate statistical significance in this subgroup.
IMMUNE CHECKPOINT INHIBITOR TOLERANCE
In a series of 8 studies that featured
patients with advanced RCC and
other metastatic solid tumors, no
association between patients with
sarcopenia and adverse reactions of
any grade were identified.84 However,
in a separate review, an increased
risk of AEs with the use of ICIs
in sarcopenic cancer patients was
observed.85 In addition to standard
TRAEs from systemic therapy,
numerous immune-related adverse
events (irAE) associated with ICI
use that result from upregulation
of the host immune system.86 The
most commonly affected organs
include the gastrointestinal tract,
endocrine glands, skin, and liver.86
Intriguingly, in a review of 90
patients with ICI-treated RCC, there
was a 42% prevalence of irAEs, and
this cohort demonstrated improved
OS compared to patients without
irAEs (35.9 [95% CI, 24.3 to non-estimable] vs 26.5 months [95%
CI, 10.2-28.8]; p=0.002).87 Similar
studies have supported the findings
of longer OS and PFS in ICI-treated
RCC patients reporting greater
irAEs.88,89 In a meta-analysis of ,
patients with advanced solid tumors,
researchers analyzed sarcopenia in
relation to irAEs, but the findings
were mixed: two of the studies found
no significant association between
sarcopenia and irAEs, however, the
3rd study did identify a higher chance
of developing irAEs in the sarcopenic
group.85 An association between
sarcopenia and grade 3-4 irAEs may
explain the lack of survival benefit
in this cohort compared to other
studies assessing the prognostic
value of irAEs.90 Although certain
studies support sarcopenia as a
risk factor for ICI TRAEs, the topic
remains controversial and study-dependent. From a pharmacokinetic
perspective, susceptibility to
TRAEs in sarcopenic patients
makes sense; however, much of the
research is limited by sample size,
retrospective nature, and inclusion
of a wide diversity of tumor types.
New prospective studies should be
pursued to examine the impact that
muscle, inflammation, and nutrition
may have ICI-related toxicity in RCC.
CONCLUSION
There remains a high prevalence of
RCC cases that are either diagnosed
at or progress to an advanced stage.
ICI-based regimens including ICIs
have emerged as first-line treatments
for patients with advanced or
metastatic disease. Measurements
of sarcopenia, inflammation and
nutrition hold potential prognostic
value for the long-term outcomes
of localized and advanced RCC.
Strategies aimed for preventing
and managing sarcopenia may have
significant impact on improving
outcomes and quality of life in
patients with metastatic RCC



REFERENCE
1. Cancer of the Kidney and Renal Pelvis - Cancer Stat Facts. SEER. Accessed
February 23, 2023. https://seer.cancer.gov/
statfacts/html/kidrp.html
2. Bukavina L, Bensalah K, Bray F, et al. Epidemiology of Renal Cell Carcinoma:
2022 Update. Eur Urol. 2022;82(5):529-542.
3. Padala SA, Barsouk A, Thandra KC, et al. Epidemiology of Renal Cell Carcinoma.
World J Oncol. 2020;11(3):79-87.
4. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in
Advanced Renal-Cell Carcinoma. N Engl J
Med. 2015;373(19):1803-1813.
5. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus
Ipilimumab versus Sunitinib in Advanced
Renal-Cell Carcinoma. N Engl J Med.
2018;378(14):1277-1290.
6. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus Axitinib versus Sunitinib
for Advanced Renal-Cell Carcinoma. N Engl
J Med. 2019;380(12):1103-1115.
7. Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus Cabozantinib
versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med.
2021;384(9):829-841.
8. Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus Pembrolizumab
or Everolimus for Advanced Renal
Cell Carcinoma. N Engl J Med.
2021;384(14):1289-1300.
9. Tykodi SS, Gordan LN, Alter RS, et al. Safety and efficacy of nivolumab plus
ipilimumab in patients with advanced
non-clear cell renal cell carcinoma: results
from the phase 3b/4 CheckMate 920
trial. J Immunother Cancer. 2022;10(2).
doi:10.1136/jitc-2021-003844
10. Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib
versus sunitinib monotherapy as first-line
treatment of advanced renal cell carcinoma
(KEYNOTE-426): extended follow-up from
a randomised, open-label, phase 3 trial.
Lancet Oncol. 2020;21(12):1563-1573.
11. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib
versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med.
2019;380(12):1116-1127.
12. Motzer RJ, Jonasch E, Agarwal N, et al. Kidney Cancer, Version 3.2022, NCCN
Clinical Practice Guidelines in Oncology. J
Natl Compr Canc Netw. 2022;20(1):71-90.
13. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet.
2019;393(10191):2636-2646.
14. Williams GR, Chen Y, Kenzik KM, et al. Assessment of Sarcopenia Measures,
Survival, and Disability in Older Adults
Before and After Diagnosis With Cancer.
JAMA Netw Open. 2020;3(5):e204783.
15. Surov A, Wienke A. Prevalence of sarcopenia in patients with solid
tumors: A meta-analysis based on 81,814
patients. JPEN J Parenter Enteral Nutr.
2022;46(8):1761-1768.
16. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer
cachexia: an international consensus.
Lancet Oncol. 2011;12(5):489-495.
17. Khan AI, Psutka SP, Patil DH, et al. Sarcopenia and systemic inflammation
are associated with decreased survival
after cytoreductive nephrectomy for
metastatic renal cell carcinoma. Cancer.
2022;128(11):2073-2084.
18. Higgins MI, Martini DJ, Patil DH, et al. Sarcopenia and modified Glasgow
Prognostic Score predict postsurgical
outcomes in localized renal cell carcinoma.
Cancer. 2021;127(12):1974-1983.
19. Psutka SP, Boorjian SA, Moynagh MR, et al. Decreased Skeletal Muscle Mass
is Associated with an Increased Risk of
Mortality after Radical Nephrectomy
for Localized Renal Cell Cancer. J Urol.
2016;195(2):270-276.
20. Sharma P, Zargar-Shoshtari K, Caracciolo JT, et al. Sarcopenia as a predictor
of overall survival after cytoreductive
nephrectomy for metastatic renal cell
carcinoma. Urol Oncol. 2015;33(8):339.
e17-e23.
21. Fukushima H, Nakanishi Y, Kataoka M, Tobisu KI, Koga F. Prognostic
Significance of Sarcopenia in Patients with
Metastatic Renal Cell Carcinoma. J Urol.
2016;195(1):26-32.
22. Maurits JSF, Sedelaar JPM, Mulders PFA, Aben KKH, Kiemeney LALM,
Vrieling A. Skeletal muscle radiodensity and
visceral adipose tissue index are associated
with survival in renal cell cancer - A
multicenter population-based cohort study.
Clin Nutr. 2022;41(1):131-143.
23. Schmeusser BN, Midenberg E, Palacios AR, et al. Clinic friendly estimation
of muscle composition: Preoperative linear
segmentation shows overall survival
correlated with muscle mass in patients
with nonmetastatic renal cell carcinoma.
Front Oncol. 2022;12:1068357.
24. Watanabe S, Ishihara H, Takagi T, et al. Impact of sarcopenia on post-operative
outcomes following nephrectomy and tumor
thrombectomy for renal cell carcinoma with
inferior vena cava thrombus. Jpn J Clin
Oncol. 2021;51(5):819-825.
25. Hofbauer SL, Pantuck AJ, de Martino M, et al. The preoperative prognostic
nutritional index is an independent
predictor of survival in patients with renal
cell carcinoma. Urol Oncol. 2015;33(2):68.
e1-e7.
26. Peng D, He ZS, Li XS, et al. Prognostic Value of Inflammatory and
Nutritional Scores in Renal Cell Carcinoma
After Nephrectomy. Clin Genitourin Cancer.
2017;15(5):582-590.
27. Gu W, Zhang G, Sun L, et al. Nutritional screening is strongly associated
with overall survival in patients treated
with targeted agents for metastatic renal
cell carcinoma. J Cachexia Sarcopenia
Muscle. 2015;6(3):222-230.
28. Schmeusser BN, Patil DH, Midenberg E, et al. Data regarding
covariates significantly associated with
sarcopenia and varying albumin statuses
in patients with renal cell carcinoma. Data
Brief. 2022;45(108724):108724.
29. Midenberg E, Higgins MI, Schmeusser BN, et al. Prognostic Value of
Sarcopenia and Albumin in the Surgical
Management of Localized Renal Cell
Carcinoma. Urologic Oncology: Seminars
and Original Investigations. Published
online October 21, 2022. doi:10.1016/j.
urolonc.2022.09.020
30. Dobosz P, Dzieciątkowski T. The Intriguing History of Cancer
Immunotherapy. Front Immunol.
2019;10:2965.
31. Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade
therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors.
Int Immunopharmacol. 2018;62:29-39.
32. Motzer RJ, Escudier B, McDermott DF, et al. Survival outcomes and independent
response assessment with nivolumab plus
ipilimumab versus sunitinib in patients with
advanced renal cell carcinoma: 42-month
follow-up of a randomized phase 3 clinical
trial. J Immunother Cancer. 2020;8(2).
doi:10.1136/jitc-2020-000891
33. Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with
pembrolizumab in patients with advanced
renal cell cancer: a non-randomised,
open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol.
2018;19(3):405-415.
34. Albiges L, Barthélémy P, Gross- Goupil M, Negrier S, Needle MN, Escudier
B. TiNivo: safety and efficacy of tivozanib-nivolumab combination therapy in patients
with metastatic renal cell carcinoma. Ann
Oncol. 2021;32(1):97-102.
35. Naing A, Gainor JF, Gelderblom H, et al. A first-in-human phase 1 dose escalation
study of spartalizumab (PDR001), an anti-PD-1 antibody, in patients with advanced
solid tumors. J Immunother Cancer.
2020;8(1). doi:10.1136/jitc-2020-000530
36. Msaouel P, Goswami S, Thall PF, et al. A phase 1-2 trial of sitravatinib
and nivolumab in clear cell renal cell
carcinoma following progression on
antiangiogenic therapy. Sci Transl Med.
2022;14(641):eabm6420.
37. Campbell MT, Matin SF, Tam AL, et al. Pilot study of Tremelimumab
with and without cryoablation in patients
with metastatic renal cell carcinoma. Nat
Commun. 2021;12(1):6375.
38. Tannir NM, Cho DC, Diab A, et al. Bempegaldesleukin plus nivolumab in first-line renal cell carcinoma: results from the
PIVOT-02 study. J Immunother Cancer.
2022;10(4). doi:10.1136/jitc-2021-004419
39. Stubbs C, Bardoli AD, Afshar M, et al. A Study of Angiogenesis Markers
in Patients with Renal Cell Carcinoma
Undergoing Therapy with Sunitinib.
Anticancer Res. 2017;37(1):253-259.
40. Brighi N, Farolfi A, Conteduca V, et al. The Interplay between Inflammation,
Anti-Angiogenic Agents, and Immune
Checkpoint Inhibitors: Perspectives for
Renal Cell Cancer Treatment. Cancers .
2019;11(12). doi:10.3390/cancers11121935
41. Ricketts CJ, De Cubas AA, Fan H, et al. The Cancer Genome Atlas Comprehensive
Molecular Characterization of Renal Cell
Carcinoma. Cell Rep. 2018;23(1):313-326.
e5.
42. Chen F, Zhang Y, Şenbabaoğlu Y, et al. Multilevel Genomics-Based Taxonomy
of Renal Cell Carcinoma. Cell Rep.
2016;14(10):2476-2489.
43. Geissler K, Fornara P, Lautenschläger C, Holzhausen HJ, Seliger
B, Riemann D. Immune signature of tumor
infiltrating immune cells in renal cancer.
Oncoimmunology. 2015;4(1):e985082.
44. Itsumi M, Tatsugami K. Immunotherapy for renal cell carcinoma.
Clin Dev Immunol. 2010;2010:284581.
45. Chevrier S, Levine JH, Zanotelli VRT, et al. An Immune Atlas of Clear Cell
Renal Cell Carcinoma. Cell. 2017;169(4):736-
749.e18.
46. Moreira M, Pobel C, Epaillard N, Simonaggio A, Oudard S, Vano YA.
Resistance to cancer immunotherapy in
metastatic renal cell carcinoma. Cancer
Drug Resist. 2020;3(3):454-471.
47. George S, Rini BI, Hammers HJ. Emerging Role of Combination
Immunotherapy in the First-line Treatment
of Advanced Renal Cell Carcinoma: A
Review. JAMA Oncol. 2019;5(3):411-421.
48. Bi K, He MX, Bakouny Z, et al. Tumor and immune reprogramming during
immunotherapy in advanced renal cell
carcinoma. Cancer Cell. 2021;39(5):649-
661.e5.
49. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European
consensus on definition and diagnosis. Age
Ageing. 2019;48(1):16-31.
50. Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose
tissue volumes: estimation from a single
abdominal cross-sectional image. J Appl
Physiol. 2004;97(6):2333-2338.
51. Higgins MI, Martini DJ, Patil DH, et al. Quantification of body composition in
renal cell carcinoma patients: Comparing
computed tomography and magnetic
resonance imaging measurements. Eur J
Radiol. 2020;132:109307.
52. Khan AI, Reiter DA, Sekhar A, et al. MRI quantitation of abdominal
skeletal muscle correlates with CT-based
analysis: implications for sarcopenia
measurement. Appl Physiol Nutr Metab.
2019;44(8):814-819.
53. Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos
VE. A practical and precise approach to
quantification of body composition in
cancer patients using computed tomography
images acquired during routine care. Appl
Physiol Nutr Metab. 2008;33(5):997-1006.
54. Steele S, Lin F, Le TL, et al. Segmentation and Linear Measurement for
Body Composition Analysis using Slice-O-Matic and Horos. J Vis Exp. 2021;(169).
doi:10.3791/61674
55. Dent E, Woo J, Scott D, Hoogendijk EO. Sarcopenia measurement in research
and clinical practice. Eur J Intern Med.
2021;90:1-9.
56. Dalle S, Rossmeislova L, Koppo K. The Role of Inflammation in Age-Related
Sarcopenia. Front Physiol. 2017;8:1045.
57. Dodds RM, Roberts HC, Cooper C, Sayer AA. The Epidemiology of Sarcopenia.
J Clin Densitom. 2015;18(4):461-466.
58. Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory
mediators in the elderly. Exp Gerontol.
2004;39(5):687-699.
59. Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr
Metab Care. 2012;15(1):12-22.
60. Li CW, Yu K, Shyh-Chang N, et al. Circulating factors associated with
sarcopenia during ageing and after intensive
lifestyle intervention. J Cachexia Sarcopenia
Muscle. 2019;10(3):586-600.
61. Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor
necrosis factor-alpha with muscle mass and
muscle strength in elderly men and women:
the Health ABC Study. J Gerontol A Biol Sci
Med Sci. 2002;57(5):M326-M332.
62. Bano G, Trevisan C, Carraro S, et al. Inflammation and sarcopenia:
A systematic review and meta-analysis.
Maturitas. 2017;96:10-15.
63. Williams GR, Dunne RF, Giri S, Shachar SS, Caan BJ. Sarcopenia in the
Older Adult With Cancer. J Clin Oncol.
2021;39(19):2068-2078.
64. Law ML. Cancer cachexia:
Pathophysiology and association with
cancer-related pain. Front Pain Res
(Lausanne). 2022;3:971295.
65. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of
sarcopenia in adults with solid tumours: A
meta-analysis and systematic review. Eur J
Cancer. 2016;57:58-67.
66. Yuxuan L, Junchao L, Wenya L. The role of sarcopenia in treatment-related outcomes in patients with renal cell
carcinoma: A systematic review and meta-analysis. Medicine . 2022;101(43):e31332.
67. Mao W, Wang K, Zhang H, et al. Sarcopenia as a poor prognostic indicator
for renal cell carcinoma patients undergoing
nephrectomy in China: A multicenter study.
Clin Transl Med. 2021;11(1):e270.
68. Medline A, Midenberg E, Patil D, et al. Muscle mass change using linear
measurement analysis after nephrectomy
for pT3 and pT4 renal cell carcinoma is
associated with mortality. JCSM Rapid
Communications. 2022;5(2):205-211.
69. Hu X, Wang Y, Yang WX, Dou WC, Shao YX, Li X. Modified Glasgow
prognostic score as a prognostic factor for
renal cell carcinomas: a systematic review
and meta-analysis. Cancer Manag Res.
2019;11:6163-6173.
70. Deng HY, Chen ZJ, Qiu XM, Zhu DX, Tang XJ, Zhou Q. Sarcopenia and
prognosis of advanced cancer patients
receiving immune checkpoint inhibitors: A
comprehensive systematic review and meta-analysis. Nutrition. 2021;90:111345.
71. Takenaka Y, Oya R, Takemoto N, Inohara H. Predictive impact of
sarcopenia in solid cancers treated with
immune checkpoint inhibitors: a meta-analysis. J Cachexia Sarcopenia Muscle.
2021;12(5):1122-1135.
72. Loosen SH, van den Bosch V, Gorgulho J, et al. Progressive Sarcopenia
Correlates with Poor Response and Outcome
to Immune Checkpoint Inhibitor Therapy.
J Clin Med Res. 2021;10(7). doi:10.3390/
jcm10071361
73. Rounis K, Makrakis D, Gioulbasanis I, et al. Cancer Cachexia and
Antitumor Immunity: Common Mediators
and Potential Targets for New Therapies.
Life. 2022;12(6). doi:10.3390/life12060880
74. Martini DJ, Olsen TA, Goyal S, et al. Body Composition Variables as
Radiographic Biomarkers of Clinical
Outcomes in Metastatic Renal Cell
Carcinoma Patients Receiving Immune
Checkpoint Inhibitors. Front Oncol. 2021;11.
doi:10.3389/fonc.2021.707050
75. Martini DJ, Kline MR, Liu Y, et al. Adiposity may predict survival in patients
with advanced stage cancer treated with
immunotherapy in phase 1 clinical trials.
Cancer. 2020;126(3):575-582.
76. Herrmann T, Mione C, Montoriol PF, et al. Body Mass Index, Sarcopenia, and
Their Variations in Predicting Outcomes
for Patients Treated with Nivolumab for
Metastatic Renal Cell Carcinoma. Oncology.
2022;100(2):114-123.
77. Ged Y, Sanchez A, Patil S, et al. Associations between Pretreatment
Body Composition Features and Clinical
Outcomes among Patients with Metastatic
Clear Cell Renal Cell Carcinoma Treated
with Immune Checkpoint Blockade. Clin
Cancer Res. 2022;28(23):5180-5189.
78. Bilen MA, Martini DJ, Liu Y, et al. Combined Effect of Sarcopenia and
Systemic Inflammation on Survival in
Patients with Advanced Stage Cancer
Treated with Immunotherapy. Oncologist.
2020;25(3):e528-e535.
79. Bilen MA, Dutcher GMA, Liu Y, et al. Association Between Pretreatment
Neutrophil-to-Lymphocyte Ratio and
Outcome of Patients With Metastatic
Renal-Cell Carcinoma Treated With
Nivolumab. Clin Genitourin Cancer.
2018;16(3):e563-e575.
80. Zahoor H, Barata PC, Jia X, et al. Patterns, predictors and subsequent
outcomes of disease progression in
metastatic renal cell carcinoma patients
treated with nivolumab. J Immunother
Cancer. 2018;6(1):107.
81. Ni L, Huang J, Ding J, et al. Prognostic Nutritional Index Predicts
Response and Prognosis in Cancer
Patients Treated With Immune Checkpoint
Inhibitors: A Systematic Review and Meta-Analysis. Front Nutr. 2022;9:823087.
82. Aslan V, Kılıç ACK, Sütcüoğlu O, et al. Cachexia index in predicting
outcomes among patients receiving
immune checkpoint inhibitor treatment for
metastatic renal cell carcinoma. Urol Oncol.
2022;40(11):494.e1-e494.e10.
83. Hurkmans DP, Basak EA, van Dijk T, et al. A prospective cohort study
on the pharmacokinetics of nivolumab
in metastatic non-small cell lung cancer,
melanoma, and renal cell cancer patients. J
Immunother Cancer. 2019;7(1):192.
84. Li S, Wang T, Lai W, et al. Prognostic impact of sarcopenia on immune-related
adverse events in malignancies received
immune checkpoint inhibitors: a systematic
review and meta-analysis. Transl Cancer
Res. 2021;10(12):5150-5158.
85. Guzman-Prado Y, Ben Shimol J, Samson O. Sarcopenia and the risk of
adverse events in patients treated with
immune checkpoint inhibitors: a systematic
review. Cancer Immunol Immunother.
2021;70(10):2771-2780.
86. Postow MA, Sidlow R, Hellmann MD. Immune-Related
Adverse Events Associated with Immune
Checkpoint Blockade. N Engl J Med.
2018;378(2):158-168.
87. Elias R, Yan F, Singla N, et al. Immune-related adverse events are
associated with improved outcomes in ICI-treated renal cell carcinoma patients. J Clin
Orthod. 2019;37(7_suppl):645-645.
88. Ishihara H, Takagi T, Kondo T, et al. Association between immune-related
adverse events and prognosis in patients
with metastatic renal cell carcinoma treated
with nivolumab. Urol Oncol. 2019;37(6):355.
e21-e355.e29.
89. Paderi A, Giorgione R, Giommoni E, et al. Association between Immune
Related Adverse Events and Outcome
in Patients with Metastatic Renal Cell
Carcinoma Treated with Immune
Checkpoint Inhibitors. Cancers . 2021;13(4).
doi:10.3390/cancers13040860
90. Liu X, Shi Y, Zhang D, et al. Risk factors for immune-related adverse events:
what have we learned and what lies ahead?
Biomark Res. 2021;9(1):79
* Corresponding Author: Rohan Garje, MD
Chief of Genitourinary Medical Oncology, Miami Cancer Institute, Baptist Health South Florida
8900 N. Kendall Drive | Miami, FL 33176 Email Id: rohan.garje@baptisthealth.net