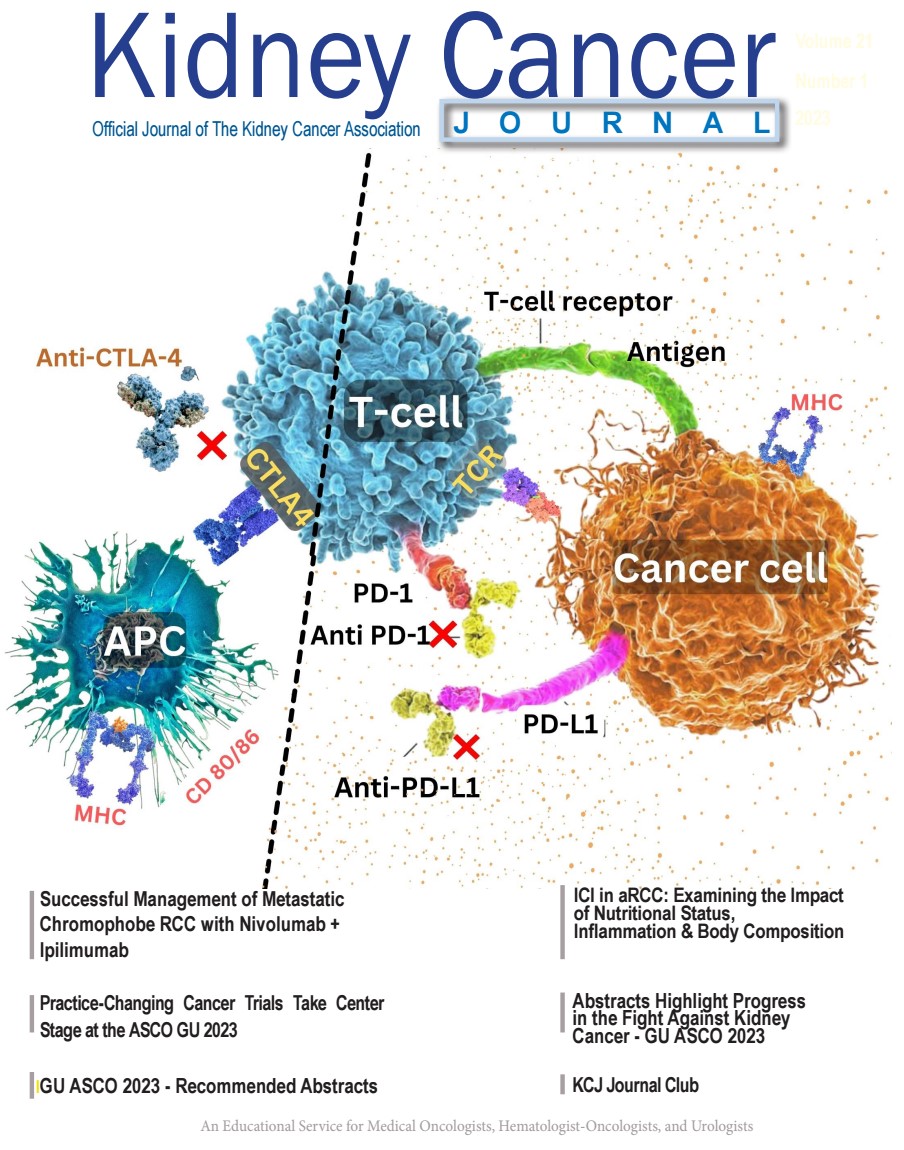
https://doi.org/10.52733/KCJ21n1-e


Practice-Changing Cancer Trials Take Center Stage at the ASCO GU 2023

Cedars Cinai Cancer Center, Los Angeles, CA
Correspondence to: Email: robert.figlin@cshs.org
Dear Colleagues,
More than 5,600 clinicians and researchers from 79 countries gathered this February 18-20 at the American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU) in San Francisco to discuss practice-changing research, novel care approaches across the spectrum of GU malignancies. It was exciting to see this year’s theme “Today’s Science, Tomorrow’s Treatment,” accurately reflected in practice-changing data, the oral and poster sessions, small group discussions, and thoughtful Q&A sessions at the conference. This year’s keynote session was delivered by Dr. Norman Sharpless, former director of the NCI. In his keynote address entitled “Ending Cancer as We Know It: Predicting Future Cancer Progress” he described the impact of the Cancer Moonshot to accelerate the rate of progress against cancer especially in the context of GU oncology. Dr. Sharpless also shared his insights on how funding allocated to Moonshot 2.0 will impact all attendees. Additionally, he highlighted how the NCI’s National Clinical Trials Network can adapt to meet the needs of the oncology community.




Some key abstracts at the conference
delivered the groundbreaking results,
novel therapies and innovations in the
GU oncology space. For example, how
artificial intelligence can be exploited to
improve digital pathology and radiology
as well as the potential for this technology to improve care.
Sessions also highlighted new clinical practice approaches to
complex care management issues across malignancies. In the
kidney cancer space, research presented at the symposium
provided follow-up or updated data from pivotal clinical
trials including Cosmic-313, COSMIC-021, KEYNOTE-564
and CheckMate 9ER. In the special section of this issue, I
have also listed some important kidney cancer abstracts
presented at GU ASCO sessions. Here is a quick recap of
some of the important findings from ASCO GU 2023.
There is an unmet need for accurate noninvasive
techniques to guide patient management. The ZIRCON study
(Abstract LBA 602) evaluated the performance of TLX250-
CDx PET/CT for detection of ccRCC in adult patients with
IDRM. Results indicated that TLX250-CDx PET/CT is well
tolerated and can accurately and noninvasively identify tumors
in ccRCC patients with IDRM. Preliminary results from the
COSMIC-313 (abstract 605) showed that adding cabozantinib
to nivolumab plus ipilimumab significantly increased the time
to when the treatment stopped working and progression-free
survival in patients with intermediate-risk kidney cancer.
The follow-up results from CheckMate-9ER (abstract 603),
shows that the combination of cabozantinib plus nivolumab
continues to improve survival, control the cancer, and shrink
the cancer on scans compared with sunitinib in patients
with advanced kidney cancer who had not previously taken
any treatment. The exploratory post hoc biomarker analysis
(Abstract 608) using the patients from the CheckMate-9ER
study indicates that PD-1 biomarker did not predict the
progression-free survival and overall survival time outcomes.
All the genetic tests did not predict patient outcomes in this
study. This suggests that key determinants of response to anti–
PD-1 vs anti–PD-L1 therapies may differ.

 The latest results from subgroup exploratory analyses
(abstract 679) of KEYNOTE-564 study confirmed that adjuvant
pembro prolonged DFS compared with pbo for all subgroups
in consistent with the results of the ITT population. This
further support the use of adjuvant pembro after nephrectomy
as standard of care for pts with RCC at increased risk of
recurrence. The extended follow-up results of the COSMIC-021
study demonstrates encouraging clinical activity of
cabozantinib plus atezolizumab in patients with nccRCC.
This follow-up reinforces the encouraging clinical activity of
cabozantinib plus atezolizumab in advanced nccRCC with
a manageable safety profile. In another study (abstract 604),
authors assessed treatment-free survival (TFS) outcomes
from the phase II study of nivolumab and salvage nivolumab
+ ipilimumab in aRCC.
The latest results from subgroup exploratory analyses
(abstract 679) of KEYNOTE-564 study confirmed that adjuvant
pembro prolonged DFS compared with pbo for all subgroups
in consistent with the results of the ITT population. This
further support the use of adjuvant pembro after nephrectomy
as standard of care for pts with RCC at increased risk of
recurrence. The extended follow-up results of the COSMIC-021
study demonstrates encouraging clinical activity of
cabozantinib plus atezolizumab in patients with nccRCC.
This follow-up reinforces the encouraging clinical activity of
cabozantinib plus atezolizumab in advanced nccRCC with
a manageable safety profile. In another study (abstract 604),
authors assessed treatment-free survival (TFS) outcomes
from the phase II study of nivolumab and salvage nivolumab
+ ipilimumab in aRCC.




Some reports highlighted emerging trends from the HIF2a inhibitors in combination with other drugs. Abstract TPS748 provided the details about the phase 3 LITESPARK-022 study (NCT05239728) that will evaluate the efficacy and safety of pembro plus belzutifan compared with placebo plus pembro as adjuvant treatment following nephrectomy in pts with ccRCC. The primary end point is disease-free survival. Secondary end points include overall survival, safety, disease recurrence–specific survival, and patient-reported outcomes. In other abstract, (TPS747), authors presented the study design of LITESPARK-024 that investigates the efficacy of HIF-2α inhibitor belzutifan with or without CDK 4/6 inhibitor palbociclib. The primary end point is ORR per RECIST v1.1 by investigator assessment and secondary end points are clinical benefit rate, DOR, PFS, OS, and safety and tolerability. Overall, the GU ASCO 2023 conference provided valuable insights and updates on the latest research in kidney cancer, which will help in developing more effective treatments for patients with this disease.