Submitted - Februray 14, 2023 | Revised March 9, 2023 | Revised Manuscript Accepted - March 15, 2023 | ePublished - March 30, 2023
https://doi.org/10.52733/KCJ21n1-a1

ABSTRACT
Chromophobe renal cell carcinoma (chRCC) is a rare histologic variant that is morphologically and molecularly distinct compared to the more common clear cell renal cell carcinoma (ccRCC). Due to the relatively lower incidence and lack of phase III trials, treatment for metastatic chRCC is often extrapolated from ccRCC. In this case report, we discuss a 58-year-old male with metastatic chRCC who was treated with nivolumab and ipilimumab and achieved a complete response. Though there are no definite predictive biomarkers, tumors that respond to checkpoint inhibitors (CPI) have a high immunogenic gene signature, high PD-L1 expression, MSI instability, or a high tumor mutational burden. Despite a comprehensive genetic profile predicting poor response to CPI, the current patient showed sustained radiologic response over three years. This case challenges the current paradigm of predicted response to CPIs in the setting of chRCC and shows that further biomarker driven research is needed to evaluate the efficacy of these agents in chRCC.
INTRODUCTION
Renal cell carcinoma (RCC) is the
eighth most common malignancy
in the United States.1
RCC can be
divided into the more common clear
cell renal cell carcinoma (ccRCC) and
non-clear cell renal cell carcinoma
(nccRCC). Chromophobe renal cell
carcinoma (chRCC) is the third most
common histologic variant of RCC,
accounting for 5% of cases.2 Although
computerized tomography (CT) is
the preferred imaging modality in
diagnosis and staging, histologic and
molecular analysis are required to
differentiate the histologic variants
of RCC. chRCC can be differentiated
by its characteristic aneuploidy with
the entire loss of chromosomes
1,2,6,10,13, and 17. The high
expression of mitochondrial gene
mutations and accumulation of
abnormal mitochondria suggest that
the organelle is important in the
pathogenesis of chRCC.3 ChRCC can
also occur in autosomal dominant
genetic syndromes such as BirtHogg-Dube’ and tuberous sclerosis
complex.3
There is limited evidence
regarding the first-line treatment
of metastatic chRCC.2 VEGFRTKIs (cabozantinib and sunitinib)
and mTOR inhibitors (everolimus)
have traditionally been utilized in
the treatment of nccRCCs due to
their proven efficacy in ccRCC.4
Nivolumab, a PD-L1 inhibitor, has
also shown promise in treating
ccRCC resistant to VEGFR-TKIs,
but there are limited evidence in the
current literature addressing their
efficacy in the treatment of chRCC.2,5
We present the case of a patient
with cabozantinib-resistant chRCC
successfully treated with nivolumab
and ipilimumab.




CASE PRESENTATION
The patient is a 58-year-old
Caucasian male who initially
presented with left flank and lower
abdominal wall pain associated with
a 30-pound weight loss over one
year. Magnetic resonance imaging
(MRI) of abdomen showed a large
left renal mass with invasion of the
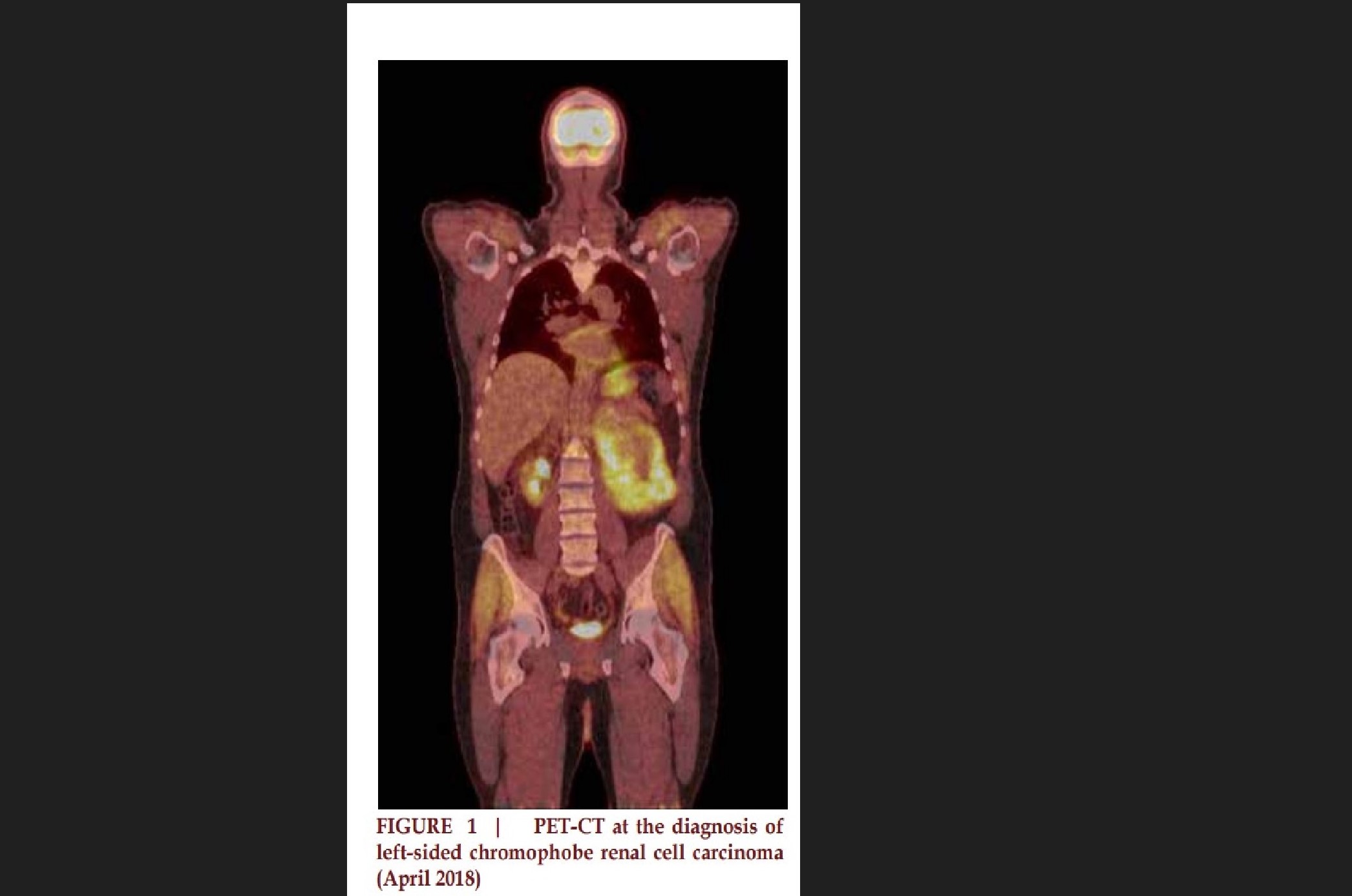
left renal vein. PET/CT confirmed
FDG avid left kidney mass. (Figure
1) Biopsy of the mass confirmed
chRCC. Subsequently, he underwent
left nephrectomy with lymph node
dissection and adrenalectomy.
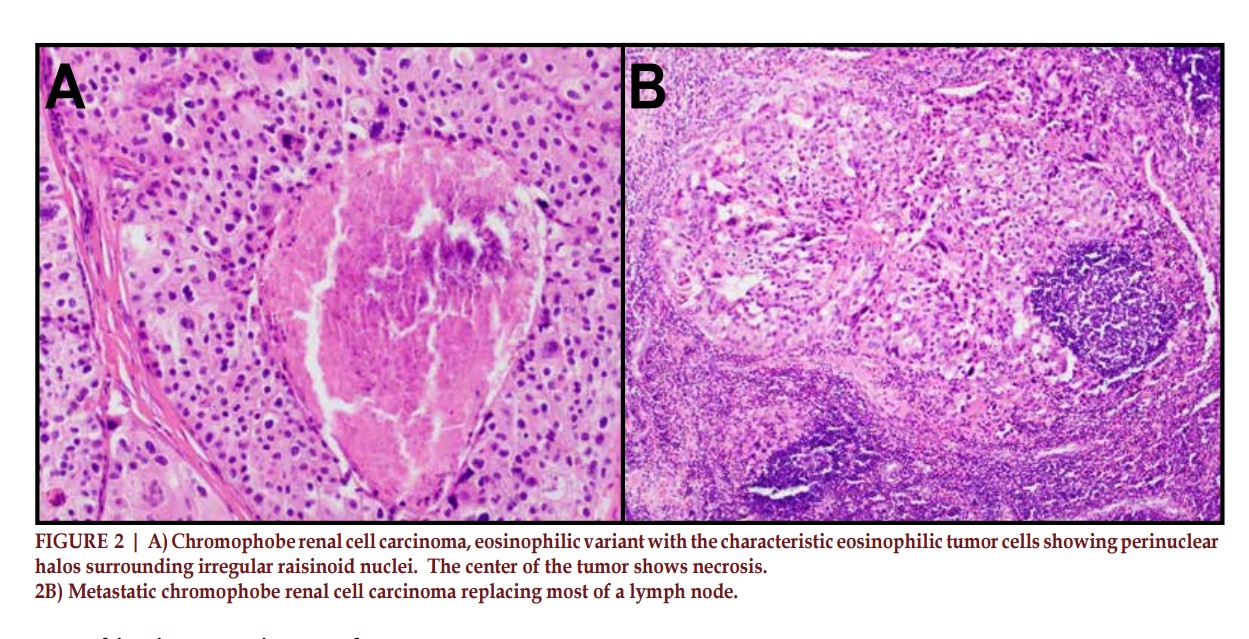
Pathology confirmed chRCC
with extensive tumor necrosis,
lymphovascular invasion, renal
sinus and perinephric fat invasion.
(Figure 2A & 2B) The surgical
margins were negative as well as
the lymph nodes and adrenal gland
were negative for metastatic disease.
Reassessment after surgery with CT
and bone scan revealed a solitary lytic
lesion in the first lumbar vertebrae,
and the patient received 30Gy/3fxs
stereotactic body radiation to the
area.
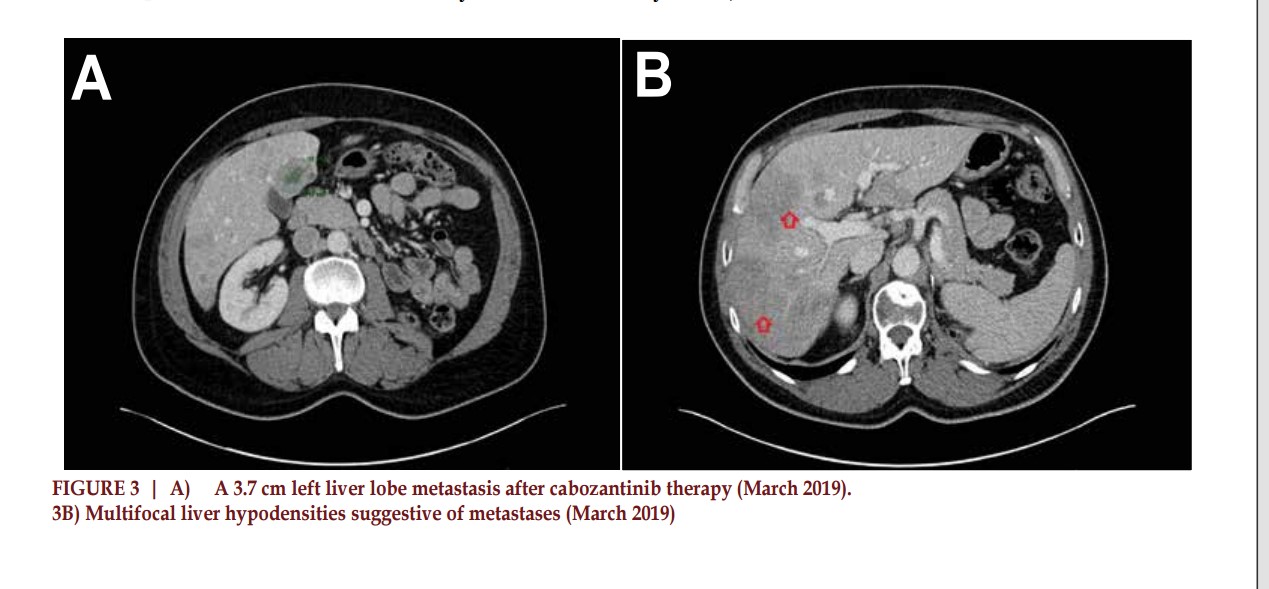
Subsequent restaging with
CT showed disease progression with
biopsy-proven liver metastases two
months after surgery, and he started
first-line systemic therapy with
cabozantinib 40 mg daily. Due to
the development of severe hand-foot
syndrome, the dose of cabozantinib
was reduced to 20 mg daily. Despite
six months of therapy, the patient
continued to have significant disease
progression, including new sites of
metastases in the lungs. (Figure 3A
& 3B). At this point in the disease
course, therapy was switched to
dual checkpoint inhibitor therapy
with nivolumab and ipilimumab.
Following the fourth cycle of this
regimen, reassessment with CT
showed partial response with
improved liver metastases and
resolution of the lung metastases.
However, immunotherapy was
discontinued after 5 months due to
development of an immune-related
adverse event (IRAE) in the form
of polyneuropathy causing Bell’s
palsy, dysphagia, and bilateral lower
extremity weakness. Brain and spine
imaging was negative for metastatic
disease or stroke. Cerebrospinal
fluid analysis showed an increase
in protein levels but was otherwise
unremarkable for infection. He was
treated with a prolonged tapering
dose of high dose prednisone with
gradual improvement of symptoms.
Despite stopping therapy after
5 months due to IRAEs, he has
ongoing complete response in the
liver, lung without any evidence of
active cancer for over 3 years now
(Figure 4). Also, he has recovered
from the IRAEs.
DISCUSSION
Although localized chRCC can be managed with surgery alone with excellent outcomes, metastatic disease requires the addition of systemic therapy with palliative intent and is generally associated with poor outcomes. The ASPEN phase II randomized control trial of 108 nccRCC patients showed everolimus, when comparable to sunitinib, showed improved overall response rate (33% versus 10% respectively).4 Within VEGFRTKIs, cabozantinib has been shown to have improved progression-free survival when compared to sunitinib in randomized controlled trials.6 After finding resistance to cabozantinib, we initiated second line therapy with nivolumab plus ipilimumab. In a retrospective analysis of 39 patients with nccRCC treated with nivolumab with or without ipilimumab, only seven patients showed objective response 6 months after therapy initiation.7 This is in comparison to the phase 3 CheckMate 214 trial that showed objective response rate of 42% in patients with ccRCC treated with nivolumab plus ipilimumab treatment in first line setting. In another review by Bersanelli et al, the objective response rates with CPIs as monotherapy or in combination with other TKIs in chRCC ranged anywhere between 0% to 28.5%.8 The studies evaluating nivolumab plus cabozantinib, atezolizumab plus cabozantinib and pembrolizumab plus lenvatinib showed objective response rates of 0%, 11% and 13.3% respectively.8 Overall the decreased responses in chRCC when compared to ccRCC can be explained by the unique molecular pathogenesis with lower PD-L1 expression, microsatellite stability, and low tumor mutational burden (TMB) in chRCC.2 Targeted genomic sequencing with FoundationOne testing which combines DNA and RNA sequencing to identify common genomic alterations and complex nucleic acid fusion events was performed on the patient’s tumor specimen. The tumor was also found to be MSI-stable with a TMB of 4 mutations per megabase. PDL1 immunohistochemical analysis revealed a tumor proportion score of 1%.
Despite the lack of any predictive markers of response to checkpoint inhibitors on the genomic profile, our patient responded well to combination immunotherapy, albeit with serious immune-related adverse events (IRAEs). A couple of retrospective studies in patients with metastatic RCC treated with CPI revealed a correlation between the incidence of IRAEs and improved oncologic outcomes.9,10 The exact mechanism underlying this association is unclear. One hypothesis is bystander effect of activated cytotoxic T-cells in an organ with low-level inflammation that is potentiated after an IRAE with CPI therapy. In particular, local inflammation caused by IRAEs may activate the immune system and lead to an increased antigen presentation, release of pro-inflammatory cytokines, and recruitment of immune cells to the tumor microenvironment. This could lead to an increased efficacy of the CPI therapy, as the immune system recognizes and responds to the tumor antigens. In a post-mortem study of patients with fulminant myocarditis secondary to CPI, T-cell receptor gene sequencing revealed similar high frequency TCRs in T cells om myocardium and tumor tissue.11 Another study revealed similar T-cell clones and antigens in the tissue obtained from the site of IRAEs and tumor.12 Though the onset of IRAE is a potential clinical marker of response to CPI, it is critical to identify those individuals at risk before therapy and understand the underlying mechanism that can aid in enhancing oncologic outcomes while minimizing serious IRAEs.
SUMMARY
In summary, while CPIs have shown some promise in the treatment of metastatic chRCC, more biomarker driven research is needed to fully understand their effectiveness in this specific subtype of RCC. Despite having low PD-L1 expression, MSI-stability, and a low TMB, our patient had a durable response with nivolumab and ipilimumab. Additional studies of nivolumab and ipilimumab are needed in a larger cohort of metastatic chRCC, along with further elucidation of mechanisms of IRAEs.
ABBREVIATION
REFERENCE
* Corresponding Author: Rohan Garje, MD Chief of Genitourinary Medical Oncology, Miami Cancer Institute, Baptist Health South Florida 8900 N. Kendall Drive | Miami, FL 33176 Email Id: rohan.garje@baptisthealth.net