Submitted - October 18, 2022 | Revised December 4, 2022 Accepted - December 7, 2022 | | ePublished - December 31, 2022
https://doi.org/10.52733/KCJ20n4-r
Treatment of Recurrent Metastatic Renal Cell Carcinoma After Adjuvant Immunotherapy
Benjamin T. Berger, MD1,*, Michael R. Harrison, MD1,2, Matthew K. Labriola, MD1,21) Department of Medicine, Duke University Medical Center, Durham, NC
2) Center for Prostate and Urologic Cancers, Duke Cancer Institute, Durham, NC
ABSTRACT
The treatment of renal cell carcinoma (RCC) has evolved dramatically in the past two decades. For metastatic RCC (mRCC), first-line treatment currently consists of vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKI), Immune Checkpoint inhibitors (ICI), or combinations of the two. In localized RCC, a recent major advancement has been the approval of the ICI pembrolizumab for adjuvant treatment of patients with a high risk of recurrence after nephrectomy. Little is known, however, regarding the optimal treatment strategy for patients with progression of disease on adjuvant therapy or recurrence after completing adjuvant therapy. Trials to inform this topic are ongoing. In the absence of this prospective data, we provide recommendations for clinicians based on existing evidence in the form of three patient scenarios. For a patient who progresses on adjuvant ICI, we generally recommend treatment with single-agent VEGFR TKI. For a patient with metastatic recurrence after completing adjuvant pembrolizumab, treatment recommendations differ based on the time from the last ICI dose until recurrence given the persistent receptor occupancy of ICI even months after discontinuation. If recurrence occurs within 6 months of the last dose of ICI, we recommend single-agent VEGFR TKI. If recurrence occurs >12 months from the last dose of ICI, we recommend resumption of ICI in combination with VEGFR TKI or dual ICI therapy. The choice between these strategies should be based on validated risk stratification instruments, time from completion of therapy, and patient-specific factors. Patients who have a recurrence within 6-12 months provide the most challenging scenario, and we would generally recommend ICI in combination with VEGFR TKI in this setting. For patients who did not tolerate adjuvant ICI, a decision on treatment with combination ICI and VEGFR TKI versus single agent VEGFR TKI should depend on the severity of the immune-related adverse event(s) resulting in intolerance as well as the time from the last dose of therapy. Individual patient considerations must also always inform treatment decisions.
INTRODUCTION
Kidney cancer is diagnosed in more
than 400,000 patients worldwide
each year1. Among kidney cancers,
greater than 90% are renal cell
carcinomas (RCC), of which
approximately 70% demonstrate
clear cell histology2. Clear cell
RCC accounts for the substantial
majority of kidney cancer morbidity
and mortality and thus has been
the subject of most kidney cancer
research. Clear cell RCC will be the
focus of this review and designated
as RCC. At the time of diagnosis,
roughly 30% of patients with RCC
will have advanced locoregional or
metastatic disease, and up to 40%
of patients initially presenting with
locoregional disease will eventually
develop metastases3. Fortunately,
great progress has been made in
the treatment of metastatic RCC
(mRCC) over the past two decades.
Median survival has increased from
approximately 15 months in the
early 2000s to greater than 4 years
in recent trials4,5.
The landscape of medical
therapies for mRCC has evolved
dramatically. Interferon (IFN) and
interleukin-2 (IL-2) were introduced
in the 1980s and 1990s6,7 and
remained the only proven systemic
therapies for over 20 years. The
VEGFR-TKI sunitinib was approved
for advanced RCC in 20068 and
revolutionized treatment. In the
following years, six additional
VEGFR TKIs were approved for
mRCC. Agents from an additional
drug class, mammalian target of
rapamycin (mTOR) inhibitors,
were also approved including
temsirolimus9 and everolimus10.
Even more recently, ICIs have
provided the next leap forward in
mRCC. Nivolumab was the first
ICI with demonstrated benefit in
mRCC11, and several subsequent
single-agent ICI trials have also
demonstrated efficacy. Multiple
options now exist for first-line
therapy in mRCC, most of which
are combinations of ICI and VEGFR
TKIs12. Important developments
have also been made in surgical and
ablative techniques for RCC and
mRCC13, 14.
The most recent major
advancement in the treatment of
RCC has been the introduction of
adjuvant ICI. The results of the
KEYNOTE-564 trial, published in
2021, showed improved disease-free survival (DFS) in localized
RCC patients treated with
adjuvant pembrolizumab after
nephrectomy15. While overall
survival (OS) data are not mature,
this practice is quickly becoming
a standard of care. It remains
unknown, however, how best to
treat patients who progress on
adjuvant therapy or recur after
its completion. Trials that will
inform management in this clinical
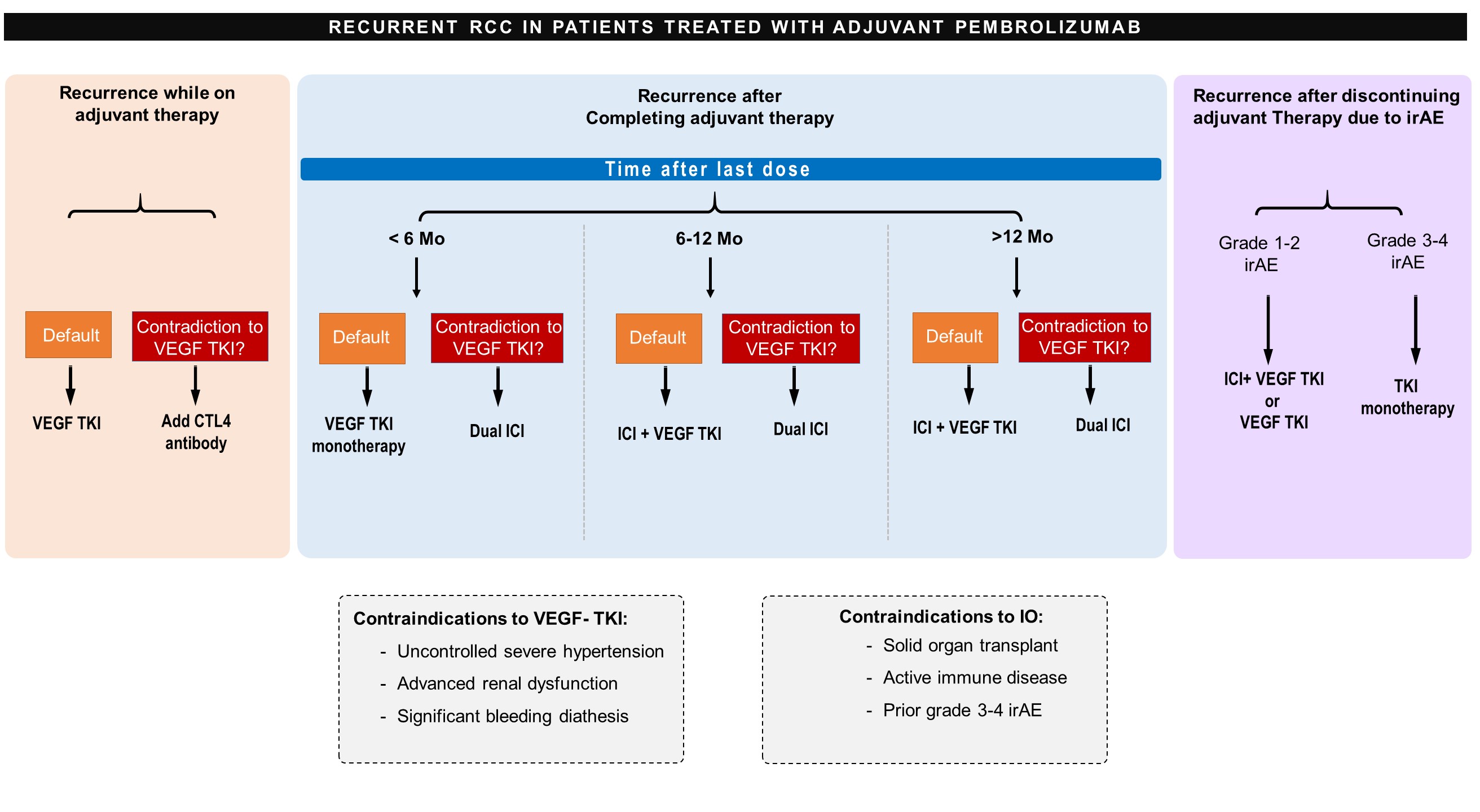
scenario are underway (Table 1)16-20. We review the current evidence
and propose a treatment algorithm
(Figure 1) to guide clinicians in
managing patients with mRCC with
recurrence on or after adjuvant
immunotherapy.
KEYNOTE-564 was the first
reported trial of ICI in the adjuvant
setting for RCC. Accrual began in
2017 and results were published in
2021 with 24 months of follow up15.
Inclusion criteria were similar to
other adjuvant trials, with eligible
patients having undergone surgery
(partial nephrectomy, nephrectomy,
and/or metastasectomy) with
negative margins but meeting
investigator criteria for high risk of
recurrence. This included patients
who were diagnosed with tumor
stage 2 with nuclear grade 4 or
sarcomatoid differentiation, tumor
stage 3 or higher, regional lymph-node metastasis, or stage M1 (distant
metastases). All patients were
disease-free at the time of trial entry
as assessed by site investigators.
Patients were randomized to
adjuvant pembrolizumab for 17 cycles
(approximately 1 year) or placebo.
The trial was positive, meeting the
primary endpoint of improved DFS
with a hazard ratio (HR) of 0.68, 95%
confidence interval (CI) 0.53 – 0.87.
At 24 months, 77.3% of patients in
the pembrolizumab arm and 68.1%
of the patients in the placebo arm
were alive and recurrence free. The
secondary endpoint of OS was also
improved (HR 0.54, 95% CI 0.30 –
0.96), with 96.6% of patients in the
pembrolizumab arm and 93.5% of
patients in the placebo arm alive
at 24 months. Grade 3 or higher
adverse events occurred in 32.4% of
patients in the pembrolizumab arm
compared to 17.7% of patients in the
placebo arm. There were no deaths
attributable to pembrolizumab or
placebo.

Notably, results of 3 different
trials of adjuvant and perioperative
ICI for RCC were published or
presented in September 2022.
The IMmotion010 trial32 was a
multicenter randomized study in
which patients with increased risk of
recurrence after nephrectomy were
treated with atezolizumab or placebo
for 1 year. The primary endpoint
of increased DFS was not met (HR
0.93, 95% CI 0.75-1.15, p=0.50). The
CheckMate 914 trial33 compared
adjuvant nivolumab plus ipilimumab
to placebo and demonstrated no
difference in the primary endpoint
of DFS (HR 0.92, 95% CI 0.71-1.19,
p=0.53). Lastly, the PROSPER
trial compared a strategy of
“perioperative” nivolumab, in which
1 dose was given prior to surgery and
9 doses were given after, to surgery
alone. This open label study was
stopped early due to futility, with
no differences in recurrence free
survival (HR 0.97, 95% CI 0.74-
1.28) or OS (HR 1.48, 95% CI 0.89-
2.48). Therefore, pembrolizumab
remains the only proven ICI agent
for adjuvant therapy.
Management of Patients with
Recurrence: Existing Guidance
No consensus exists, however,
regarding the optimal management
of patients with recurrence
during or after adjuvant ICI. This
novel category of patients may
be increasingly encountered by
clinicians given the United States
Food and Drug Administration
(FDA) approval of adjuvant
pembrolizumab in November of
202134 and ongoing trials that may
expand the use of ICI in this setting19.
In the most recent guidelines
from the National Comprehensive
Cancer Network (NCCN), published
in 202221, guidance is given for
patients considered to have relapsed
disease. However, this category
is directed at patients who have
progressed through first line
therapy for mRCC. Given the novelty
of adjuvant ICI, however, there is no
data specific to patients with disease
recurrence either on or after adjuvant
therapy. Considerations include that
adjuvant pembrolizumab is dosed
for a fixed period of 1 year, not based
on tolerability and clinical response
as in metastatic disease, and that
pembrolizumab may have a long
period of receptor occupancy after
discontinuation. Pharmacokinetic
studies of nivolumab show that
in a patient who receives at least 3
doses, the drug continues to occupy
40% of T cell PD-1 receptors for
nearly 9 months35. Similar receptor
occupancy data for pembrolizumab
are not readily available, but we
speculate that similar prolonged
binding may occur given the
similarity in their mechanisms,
terminal half-life, and clearance36.
Therefore, patients treated with
pembrolizumab in the adjuvant
setting may be managed differently
based on the timing of their
recurrence.
SCENARIO 1: Patients with
Recurrence On Adjuvant
Immunotherapy
For patients who have disease
recurrence while receiving
adjuvant ICI, we favor treatment
with single agent VEGFR TKI.
In the KEYNOTE-564 trial,
approximately 15% of patients
randomized to adjuvant therapy
had recurrence during the 12 month
period during which they were
receiving pembrolizumab. While
this scenario would appear to be
relatively uncommon based on these
data, clinicians may increasingly
encounter such patients as use of
adjuvant ICI expands and more
variable populations are treated in
real world settings.
Given the two mainstays
of mRCC treatment are either
targeting the immunogenic tumor
microenvironment or angiogenesis, it
is reasonable to target an alternative
mechanism if patients were to
progress while receiving ICI, as the
ICI clearly was not controlling the
disease. Prospective data supports
the approach of using single agent
VEGFR TKI after progressing with
prior ICI. In a phase II single-arm
study of axitinib for patients who
had previously been treated with
ICI, an overall response rate (ORR)
of 38.7% was observed37. These
were all partial responses. Among
the 40 patients included in the trial,
63% had been most recently treated
with nivolumab monotherapy. These
patients differ from our proposed
population, however, in that 71% had
received two or more prior therapies
before enrollment.
Additional prospective data
demonstrating efficacy of VEGFR
TKI after prior treatment with ICI
can be found in subgroup analyses38
of the METEOR trial39, which
randomized patients with advanced
RCC after prior antiangiogenic
therapy to cabozantinib vs
everolimus. Among 18 patients who
had also received anti-PD-1 or PD-L1 therapy and were subsequently
treated with cabozantinib, an
objective response was observed in
4 patients (22%). No responses were
seen among the 14 patients with
prior VEGFR TKI and ICI therapy
who were randomized to everolimus.
Retrospective data also
support that cabozantinib is
effective in patients who have
progressed after receiving ICI.
In a retrospective analysis of 86
patients who were treated with
cabozantinib monotherapy after
progression on ICI40, an ORR of
36% was observed. These were all
partial responses. Of the patients
included in the trial, 64% had been
previously treated with ICI alone,
while 36% had received combination
therapy with ICI and either VEGFR
TKI or another therapy. The median
number of prior therapies in these
patients was 2, with a range of 1-10.
Similar efficacy appears
to be preserved across different
agents in the VEGFR TKI class. A
retrospective study of 70 patients
who progressed after first-line
ICI therapy included patients who
were subsequently treated with
axitinib, cabozantinib, pazopanib,
or sunitinib41. An ORR of 41.2%
was observed, with 1 complete
response. These patients are similar
to those currently being treated
with adjuvant ICI in that their first
systemic therapy is an ICI. Thirty-six
percent of these patients, however
received combination therapy with
ICI + VEGFR-TKI.
There are also data to
suggest that patients who receive
a VEGFR TKI after progression on
ICI may have better outcomes if not
previously treated with a VEGFR
TKI, which may be attributable
to acquired TKI resistance. A
retrospective analysis was conducted
of 68 patients from clinical trials
who received VEGFR TKI therapy
after ICI with or without VEGFR
TKI42. Patients who previously
received a VEGFR TKI had an
ORR of only 10% with VEGFR TKI
rechallenge, while patients treated
only with ICI had an ORR of 36%,
a difference that was statistically
significant (P = 0.039). The insight
from this study may allow for more
optimistic interpretation of other
data regarding patients treated with
VEGFR TKI after ICI. Many of these
patients had previously received a
VEGFR TKI, and might have had a
better response if previously treated
with ICI alone, similar to the patients
receiving adjuvant ICI.
It is unclear whether patients
who have progressed on ICI would
benefit from continued ICI in
addition to VEGFR TKI. Based on
pre-clinical studies, it is understood
that VEGFR TKI therapy may
reverse immunosuppression in the
RCC tumor microenvironment,
promoting an immune-permissive
state and improving the efficacy
of ICI43. Data from the phase 2
KEYNOTE-146 trial44 show that
55.8% of patients previously treated
with ICI responded to lenvatinib
plus pembrolizumab, which is an
impressive post-ICI ORR. However,
57% of patients had grade 3 or
higher immune related adverse
event (irAE). This knowledge raises
the question of whether patients
receiving VEGFR TKI therapy
after progression on adjuvant
pembrolizumab would still benefit
from continuing ICI.
For patients with
contraindications to VEGFR
TKIs, the addition of an anti-CTLA-4 antibody to ICI can also
be considered. In the TITAN-RCC
trial45, patients with intermediate
and poor risk advanced RCC were
initially treated with nivolumab,
and those with early significant
PD or non-responders at 16 weeks
received “boost” cycles of nivolumab
plus ipilimumab. Of 28 patients who
received ipilimumab boosts for PD
on first-line nivolumab, 3 (11%) had
a PR and 8 (29%) achieved stable
disease.
Additional insight will
be gained from ongoing trials
evaluating the safety and efficacy
of ICI + VEGFR TKI in advanced
RCC patients with progression on
or after therapy containing ICI.
CONTACT-03 is a randomized phase
III study assessing cabozantinib plus
atezolizumab versus cabozantinib
monotherapy following progression
on or after ICI in advanced RCC16.
TiNivo-2 is a randomized phase
III study comparing tivozanib plus
nivolumab to tivozanib monotherapy
in a similar patient population17.
Estimated study completion dates
are December, 2024 and August,
2025, respectively. Lastly, PDIGREE
is an adaptive trial in which patients
with intermediate or poor risk RCC
will receive induction therapy with
ipilimumab and nivolumab and if
noted to have progressive disease
after 3 months, will be switched
to cabozantinib monotherapy.
We eagerly await the results of
these important trials, but until
then, we recommend VEGFR TKI
monotherapy for those who progress
on ICI to avoid the known toxicity
that comes with combination therapy
in the setting of unknown benefit.
SCENARIO 2: Patients with
Recurrence After Completion of
Adjuvant ICI Therapy
In KEYNOTE-564, adjuvant
pembrolizumab was given for a
maximum of 1 year (17 cycles of
doses every 3 weeks). In follow-up
data published in September 2022,
approximately 12% of patients who
did not have recurrence while on
adjuvant therapy went on to have
recurrence in the next 18 months46.
For patients that recur after the
completion on adjuvant ICI therapy,
we favor treatment selection based
on the International Metastatic
RCC Database Consortium (IMDC)
risk score as outlined in the NCCN
guidelines for first line treatment
of mRCC as well as the time until
recurrence.
In favorable risk disease,
the NCCN guidelines currently
list several combinations of ICI
plus VEGFR TKI with category
1 recommendations (defined
as being based on high level
evidence with uniform consensus
amongst committee members).
Active surveillance can also be
considered in select patients47,48
as well as single agent TKI8 for
those with contraindications
to ICI, such as uncontrolled
autoimmune disease or solid organ
transplant. In intermediate-to-high risk disease, dual ICI and
combination ICI with VEGFR TKI
are category 1 recommendations.
Multi-disciplinary discussion
of local treatment with repeat
metastasectomy or radiation
therapy can also be considered in
select patients with oligometastatic
disease.
Beyond IMDC risk
stratification, clinicians may select
the initial regimen based on the
speed with which a response is
needed, comorbid conditions,
and toxicity profile, among other
factors. For patients in whom a
more rapid response is desired, such
as those with impending visceral
crisis or very high tumor burden,
combination ICI with VEGFR TKI
would be preferred over dual ICI
given the generally accepted faster
response observed with TKIs .
For patients with recent hemorrhagic
events, uncontrolled hypertension,
or severe kidney disease, dual ICI
may be favored over combination ICI
with VEGFR TKI. Lastly, clinicians
often prioritize the chance of a
complete response and the potential
of discontinuing therapy at some
point in the future (with resulting
improved quality of life), which may
favor dual ICI therapy50.
Another factor that will
influence therapeutic decision
making is the time from completion
of therapy to metastatic recurrence.
While the half-life of pembrolizumab
has been reported at 12-26 days35,51,
indicating that most drug should
be cleared within approximately 4
months, receptor occupancy data for
the similar drug nivolumab suggests
that PD-1/PD-L1 inhibitors may
remain bound to their targets for
considerably longer. In patients who
received multiple doses, nivolumab
appeared to occupy 70% of T-cell
PD-1 receptors at 2 months, and
remained bound to 40% of receptors
for nearly 9 months. No receptor
occupancy was observed by 1 year
after the last dose35. Similar receptor
occupancy data for pembrolizumab
is not readily available. It is also
unknown to what degree receptor
occupancy translates into clinical
efficacy.
Furthermore, the duration
of ongoing immune activation
after exposure to pembrolizumab
remains unknown. There have been
rare reports of delayed immune
related adverse events (DIRE)
occurring after discontinuation
of ICI, with a systematic review of
such cases suggesting a median
interval to diagnosis of 6 months
after the last dose52. It is unclear
whether the ICI was physiologically
active at those times, or whether
an autoimmune process had been
initiated earlier in the treatment
course. The overall absence of
evidence regarding duration of ICI
activity limits our recommendations
to expert opinion. Based on existing
data and clinical experience, we
consider 12 months after the last
dose to be a time point at which the
ongoing effect of pembrolizumab is
clinically insignificant. Therefore, in
patients with recurrence 12 months
or longer after completing adjuvant
therapy, we recommend either ICI
with VEGFR TKI or dual ICI therapy
based on IMDC risk stratification
and patient specific factors. For
patients who have metastatic
recurrence within the first 6 months
of completing adjuvant therapy, we
consider the patient to have recurred
while checkpoint inhibition is
ongoing and recommend VEGFR
TKI monotherapy. For patients
with recurrence 6-12 months after
completing adjuvant therapy, it is
unclear if the ICI remains active
and thus, we generally recommend
VEGFR TKI in combination with
ICI, although TKI monotherapy or
dual ICI could be considered based
on patient specific factors. The
results of CONTACT-03, TiNivo-2,
and PDIGREE will further inform
whether additional ICI with VEGFR
TKI might benefit patients with early
relapse after completing adjuvant
therapy.
Although patients who have
received adjuvant pembrolizumab
have had exposure to the immune
targeted approach, retrospective
data indicates that treatment with
dual ICI may still be effective in
patients who have received prior
ICI. Similar efficacy (ORR 20%) was
observed in a retrospective study of
49 patients who received dual ICI
after progression on prior ICI53.
The time from last ICI treatment
appeared to be longer in patients
who responded to this “salvage”
approach, which suggests a
sensitization of tumor to ICI over time
or may simply reflect less aggressive
underlying disease. The applicability
of efficacy data from these studies to
the post-adjuvant setting, however,
is limited by the heterogeneity of
first line ICI therapies that patients
received. A variety of anti-PD-1/
PD-L1 antibodies were employed,
and often in combination with anti-CLTA-4 antibodies or other targeted
therapies.
Of note, an argument can
be made for using single agent
ICI at recurrence. A retrospective
study evaluated the outcomes of 69
patients with mRCC who received
at least 2 separate lines of ICI54.
The ORR to a second line of ICI was
23%. Importantly, response rates
did not appear to differ whether
patients received second line therapy
consisting of single agent ICI, dual
ICI, or ICI + targeted therapy. Among
the 15 patients who responded to
second line therapy 7 (46%) received
single agent ICI alone, compared to
5 (33%) who received dual ICI and
3 (30%) who received ICI + targeted
therapy. While adverse effects were
reported in total and not stratified
according to the composition of
second line therapy, this data
suggests that rechallenge with single
agent ICI may be reasonable from
the perspectives of both efficacy and
resource stewardship. However, this
is a small study, and given the robust
data for combination therapy in the
first line treatment of mRCC, we still
recommend combination therapy if
possible based on patient factors.
SCENARIO 3: Patients Who
Do Not Complete Adjuvant
Immunotherapy Due to Toxicity
In KEYNOTE-564, 8.9% of
patients randomized to adjuvant
pembrolizumab did not complete the
trial regimen, with adverse events
cited as the most common reason for
discontinuation (21.3%). For those
who discontinue treatment and have
subsequent metastatic recurrence,
the decision on a treatment regimen
should depend on the severity of
the irAE in addition to time until
recurrence, IMDC risk stratification,
and patient specific factors. We agree
with the NCCN guidelines regarding
the management of immunotherapy-related toxicities55. In general,
patients who have non-endocrine
grade 3 or 4 irAEs should not be
re-challenged with ICI and those
who have return of toxicity upon ICI
re-challenge should permanently
discontinue ICI. In patients with
grade 3 or 4 irAEs from adjuvant
pembrolizumab, we favor treatment
with single agent VEGFR TKI as in
patients who progressed on adjuvant
pembrolizumab.
For patients with
contraindications to VEGFR TKIs,
a retrospective study suggests
that ICI rechallenge may be safe
and reasonably efficacious. In
499 patients with advanced RCC
who received ICI, 71% patients
experienced an irAE. Of patients
who were given ICI in their second
line of therapy, only 45% experienced
an irAE. Similarly, grade 3 or higher
irAEs were observed in 26% and 16%
of the patients during their first and
second lines of ICI, respectively. Even
patients who experience clinically
significant irAEs may have a safe
and efficacious ICI re-challenge
therapies. Among 80 patients whose
ICI treatment was interrupted due
to an irAE, 36 (45%) were again
treated with ICI, and only 7 (19%)
experiences a grade 3 or higher
irAE (56). These data are biased
in that fewer patients with irAEs
leading to hospitalization or steroid
treatment were later rechallenged
with ICI. Among those who were
retreated, however, ICI appeared to
be moderately effective with an ORR
of 34%.
Given the pharmacokinetics
of pembrolizumab and these safety
data, we would re-challenge patients
with ICI if they recur 12 months
or more after discontinuation. ICI
plus VEGFR TKI as enumerated in
the NCCN guidelines for first line
treatment or mRCC would be favored,
and VEGFR TKI monotherapy could
also be considered. For patients
with progression in less than 6
months after ICI discontinuation
after an irAE, we would recommend
treatment with VEGFR TKI
monotherapy. For patients with
recurrence between 6-12 months,
the severity of the irAE, the IMDC
risk, and patient specific factors
would guide a more individualized
approach.
CONCLUSION
With the FDA approval of
pembrolizumab for adjuvant
treatment of localized RCC with high
recurrence risk, decision making
surrounding treatment of metastatic
recurrence is challenging.
In the absence of significant
prospective data or treatment
guidelines, we provide
recommendations for clinicians
based on existing evidence.
In general, for patients who
progress while on adjuvant ICI, we
recommend treatment with single
agent VEGFR TKI. For patients
with recurrence after completing
adjuvant pembrolizumab, we
recommend resumption of ICI with
either combination ICI and VEGFR
TKI, or dual ICI based on IMDC risk,
time from completion of therapy (<6,
6-12, or >12 months), and patient
specific factors. For patients who did
not tolerate adjuvant ICI, decision
on treatment with combination
ICI with VEGFR TKI versus single
agent VEGFR TKI is dependent on
the severity of the irAE and time
from discontinuation of therapy.
Results from ongoing clinical trials
and future prospective clinical trials
are necessary to determine the
best treatment strategies for these
patients.
FUNDING
This review did not receive any
specific grant from any funding
agency in the public, commercial, or
not-for-profit sector.
ONLINE CONTENT
Full online contents with additional
information can be accessed at
https://kidney-cancer-journal.com/
KCJ20n4-r1.php
REFERENCE
* Corresponding Author: Benjamin T. Berger, MD 200 Trent Drive, Durham, NC 27710. benjamin.t.berger@duke.edu