Submitted - September 9, 2022 | Accepted - September 20, 2022 | | ePublished - September 27, 2022
https://doi.org/10.52733/KCJ20n3-a1
Optimal Duration of Therapy in Metastatic RCC: Exploring Treatment-Free Survival with Checkpoint Inhibitors
Grayce N. Selig, MD1 Christopher J. Hoimes, DO,2 Joe Bible, PhD,3 Daniel J. George, MD,2 Michael R. Harrison, MD21. Duke University Medical Center
2) Duke Cancer Institute Center for Prostate and Urologic Cancers
CORRESPONDENCE:
ABSTRACT
The optimal duration of treatment for patients with metastatic renal cell carcinoma (mRCC) on dual immune checkpoint inhibitor (ICI) therapy remains unknown. However, there is evolving evidence that a portion of patients who achieve a complete or partial response will have a durable response, even after therapy discontinuation, leading to a prolonged treatment free survival (TFS). TFS with dual ICI is a phenomenon not seen with targeted agents and has the potential to improve patient reported outcomes and quality of life, without altering overall survival (OS). Despite this understanding, treatment of mRCC remains lifelong, as there has yet to be a prospective, randomized control trial to evaluate this key question. In this review, we analyze available studies in patients with mRCC on dual ICI therapy and propose considerations for early treatment discontinuation. Additionally, we discuss vital questions and next steps to help physicians and patients navigate these challenging treatment decisions.
INTRODUCTION
E ach year there are approximately 79,000 new cases of kidney
cancer in the United States 1
. This
number has steadily risen since early 1990s, at least in part due to more
sensitive imaging techniques. Over
the last 10 years the number of new
kidney and renal pelvis cases has increased by 0.6%, though death rates
over this period have fallen by 1.6%
2. Nevertheless, despite our diagnostic and therapeutic advances, kidney
cancer ultimately results in about
13,920 deaths per year in the United States1
. Over the last 20 years,
treatment of metastatic renal cell
carcinoma has drastically changed
resulting in prolonged survival. Systemic therapeutic options now include immune checkpoint inhibitors
(ICI) and targeted therapies (TT) in
combination or sequence based on
Phase III clinical trials demonstrating an overall survival advantage.
Most of these studies were designed
for treatment to continue indefinitely, until disease progression or unacceptable toxicities. Historically, this
approach made sense since most
patients progressed or developed
unacceptable toxicities by year two.
However, in the setting of immune
checkpoint inhibitors, a substantial percentage of patients tolerate
therapy without disease progression
for several years. By protocol, these
patients should continue therapy indefinitely, but is that necessary? To
date, few if any studies have been
designed to address this question.
Prognosis and Phases of Overall Survival.
As we continue to investigate novel biomarkers to help predict how patients may respond to therapy, many other factors, both patientand disease-specific, should be examined to help determine optimal treatment duration. Overall survival is considered the gold standard when evaluating new therapeutics in RCC. However, in patient-centric oncologic care, other endpoints are also important to consider. Overall survival can be broken down into three distinct phases: time on therapy, treatment-free survival (TFS) and time on subsequent therapy or death (Figure 1). In the targeted therapy era, monotherapies were typically sequenced, with little TFS, since outcomes were linked to dose intensity; however, in the ICI era, there may be an opportunity for meaningful TFS without compromising OS3. Critically evaluating these intervals are of the utmost importance when determining the optimal treatment strategy. Median overall survival for intermediate/poor risk mRCC has dramatically improved with the use of combination therapy, with OS approaching 47 months with dual ICI and 37.7 months with nivolumab plus cabozantinib4,5. Despite these significant advancements, a large majority of this time is spent in the clinic, between lab draws, scans, provider visits and infusion appointments. This does not account for any unplanned hospital admissions to address severe adverse events. Time spent interacting with the healthcare system, in addition to the potential for a wide spectrum of side effects, limits the quality of life(QOL). Identifying a finite treatment duration, without reducing OS, would provide patients with the needed balance between maintaining an adequate QOL outside of the hospital while continuing to battle their disease.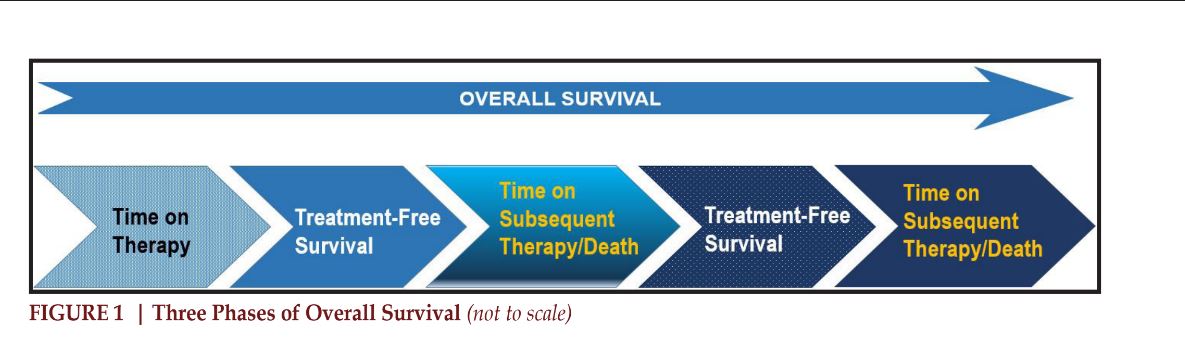
.
Immunotherapy for mRCC
The systemic treatment landscape for metastatic renal cell carcinoma has been dramatically changed by the advent of immunotherapy, initially with nivolumab (N), a PD-1 inhibitor and later with combination therapy including PD- (L)1 inhibitors with TKI as well as dual immune checkpoint inhibitor therapy. Based on the groundwork laid by CheckMate 025, 016 and 214, ipilimumab (I) and nivolumab (N) are currently the only combination immunotherapies approved in the metastatic, treatment-naïve intermediate and poor-risk (I/P) setting. However, the optimal duration of maintenance therapy with N has yet to be elucidated. This vital piece of information is critical, yet there is no robust data to predict who will respond to treatment and when to consider treatment discontinuation.CheckMate 025 was the first phase III study to evaluate singleagent nivolumab versus everolimus in patients with previously treated metastatic RCC. Nivolumab demonstrated an improvment in OS and toxicity profile when compared to everolimus6. Given the benefits seen with ICI monotherapy, CheckMate 016, a phase I study, evaluated the efficacy and safety of dual ICI with ipilimumab and nivolumab (I+N) in the first-line setting. Patients were randomized into three treatment arms to evaluate varying dosing schema, ultimately concluding that N at 3 mg/kg plus I at 1 mg/kg provided similar ORR and 2-year-OS to other dosing regimens, while minimizing toxicity7. Based on these results, a larger, randomized phase III multicenter placebo control study, CheckMate 214, began enrolling patients with previously untreated, I/P, and metastatic RCC8. Patients were randomized to either N at 3 mg/kg plus I at 1 mg/kg every 3 weeks for 4 doses followed by N at 3mg/kg every 2 weeks (I+N) or sunitinib (S), continued until disease progression or unacceptable toxicity. Notably, a protocol amendment was made 3 years into data collection, which allowed for nivolumab discontinuation at 2 years in the absence of progression or toxicity. Initial 18 month follow-up demonstrated improved median progression-free survival (PFS), overall response rate (ORR), treatment-free survival (TFS), median overall survival (OS) and patient-reported outcomes (PRO) in those treated with I + N vs S8.
Treatment with dual immune checkpoint inhibitor (ICI) therapy has improved overall survival for patients with metastatic RCC, with about 10% of patients achieving a durable complete response (CR), and another 28% achieving a partial response (PR) with varying degrees of tumor shrinkage8. There are many theories as to why patients have such a heterogeneous response to ICIs, with two possibilities involving the “cancer-immune set point” and tumor microenvironment (TME). The “cancer-immune set point” is defined as the equilibrium between anti-tumor immunity promoters and suppressors. A certain threshold must be surpassed for a patient to optimally respond to immunotherapy. This “set-point” is felt to vary widely between patients, and likely contributes to the heterogeneous treatment responses9. This equilibrium can wax and wane overtime, reflecting the tumor’s development of novel resistance patterns. In such cases, the continued priming of the immune system with ongoing therapy may be vital to maintain a durable response. Varying dosing schema is currently under investigation10,11. The presence of immune cell infiltration in the tumor and the surrounding microenvironment are also thought to be necessary, though not sufficient, to achieve a response to ICI. Checkpoint inhibitor therapy is known to decrease T cell exhaustion and promote the conversion to effector and memory T cells, which is likely necessary to achieve a durable treatment response despite treatment discontinuation9. This unique durable response has not been seen with other cancerdirected therapies and has allowed physicians to consider treatment discontinuation; allowing patients to benefit from a prolonged treatmentfree survival.
Definition of Treatment-free Survival and Key Questions Treatment-free survival (TFS) is an important metric to understand how patients live with their cancer. TFS is defined as the time from treatment discontinuation until the start of subsequent therapy or death. While overall survival is the gold standard to determine optimal therapy, treatment-free survival should not be overlooked, as it almost certainly leads to improved financial, physical, and psychological burdens that come along with chronic monthly infusional therapy. The key question is, what treatment-free interval is meaningful to patients? If overall survival is similar, would a prolonged treatment-free survival be appealing, or would it simply promote increased anxiety and fear of recurrence? These important questions will need to be explored further in subsequent studies to help physicians and patients make important treatment decisions.
TFS in CheckMate 214
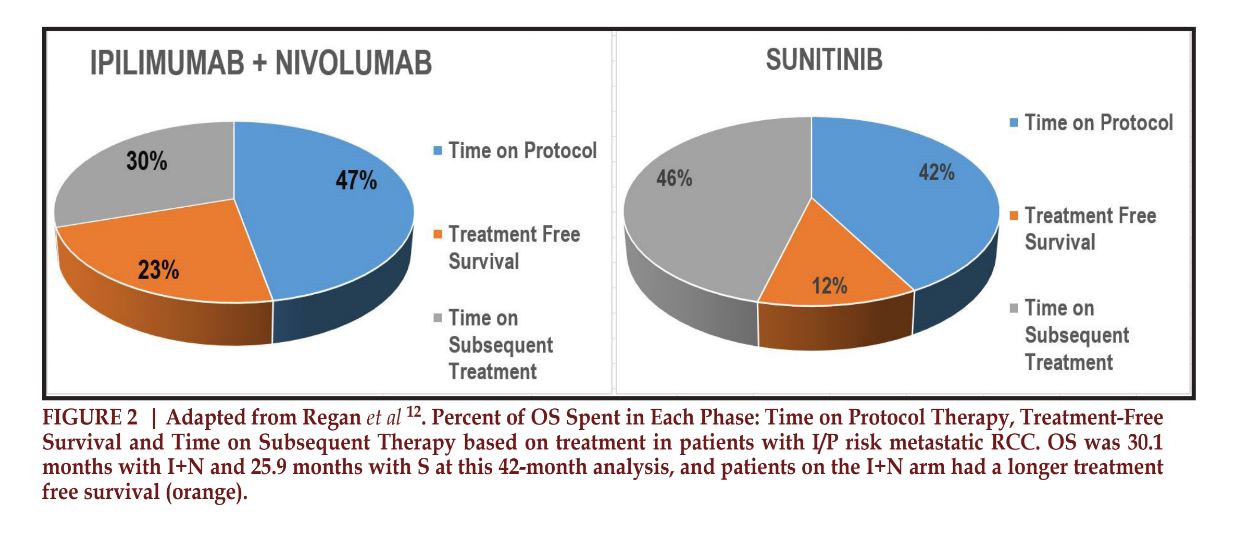
The treatment-free survival has been evaluated as a secondary endpoint in numerous studies. One such study included work by Regan et. al, who sought to evaluate the TFS following the discontinuation of therapy in patients with I/P risk disease treated on CheckMate 214. Treatment-free survival and overall survival were evaluated at 42 months. At time of evaluation 20% of patients treated with a dual immune checkpoint inhibitor (ICI) compared to 9% treated with sunitinib were treatment-free. Over the 42-month, the mean TFS and OS for patients with I/P risk mRCC was significantly longer when treated with I+N vs S, 6.9 (22.9% of OS) and 30.1 months versus 3.1 months (11.9% of OS) and 25.9 months respectively (Figure 2). In the favorable risk population, TFS was even longer, 11.0 months vs 3.7 months12. When TFS was further broken down, it was significantly longer for patients who had an objective response to therapy and even longer in those who achieved a CR, a median (range) of 23.5 months and 34.6 months (0.5-49.7 months) respectively 13, 14. Ongoing studies across risk groups are aimed at predicting who is mostly like to objectively respond to treatment.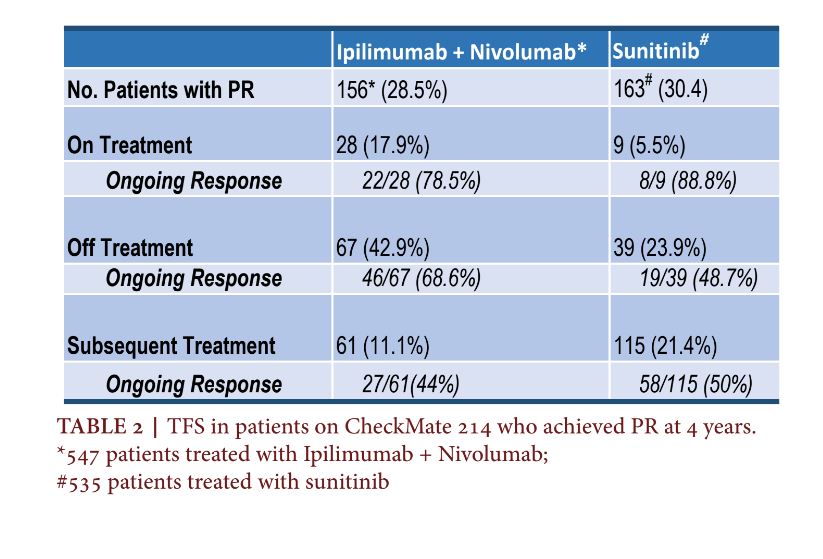
The median treatment duration reported in the 42-month analysis in the I/P risk population was 14.1 months on I+N versus 10.8 months on S. Responders remained on therapy longer, with a median duration of treatment of 20.6 months (17.7-23.2) and 21.2 months (18.9-24.4) for the I+N and S cohorts respectively 8, 12, 15. Despite differing time on protocol therapy, the median time between treatment discontinuation until death was similar, at 16 months on I+N vs 15.1 months on S; but the differences were seen in the percentages of patients reaching a TFS, with 43% vs 20% of patients recording a TFS for I+N vs S respectively (Figure 2). When critically evaluating the TFS in patients treated on CheckMate 214, one must take into consideration that the initial protocol did not allow discontinuation of therapy until disease progression or TRAE until an amendment almost 3 years into the trial. Presumably, there are a portion of patients with CR/PR who could have stopped therapy at 2 years, or earlier, if protocol allowed, which would have further prolonged the treatment-free survival 12.
Since the initial publication of CheckMate 214, updated analyses have been performed. At 4 years since randomization (median follow up 55 months), 53 (10%) of 547 patients in I+N arm and 15 (3%) of 535 patients in S arm were continued on therapy. The median OS in I/P risk groups was an impressive 48.1 months with I+N vs 26.6 months for sunitinib (HR 0.65; 95% CI, 0.54- 0.78). Dual ICI demonstrated a fouryear OS probability of 50%, vs 35.8% with sunitinib (53.4% vs 43.3% in the ITT population)4. Five-year data was recently published (median follow-up 67.7 months), which again confirmed superior OS for I/P risk patients with I+N vs S, median OS 47.0 vs 26.6 months (HR 0.68 and 95% CI 0.58 to 0.81), respectively. Five-year OS probabilities were 43% on I+N versus 31% on S16,17. Responders to I+N appeared to have decreased disease burden and higher PD-L1 expression as compared to nonresponders, with 75% of responders achieving an objective response by 4 months 15.
TFS with Complete Response (CR).
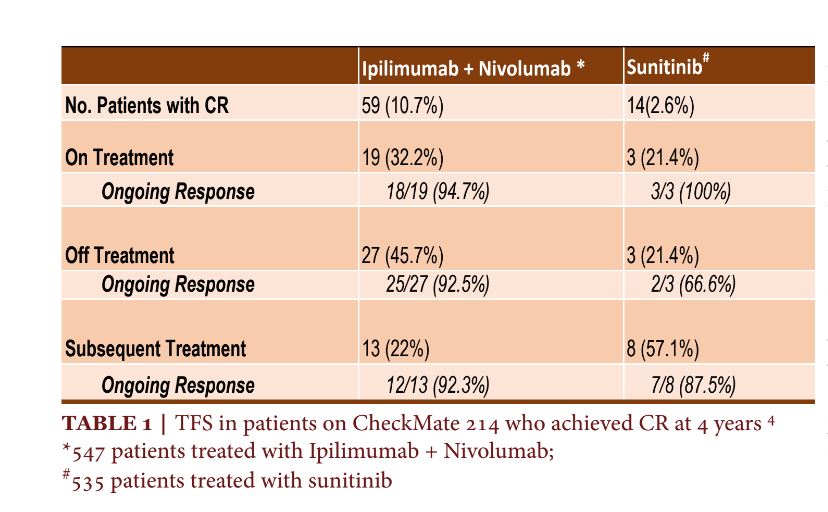
At 4 years, 10.7% (59) of patients achieved a complete response on I+N, with over 75% of these responses occurring by 11.3 months (3.8-15.4). Most converted from a PR (75.9%) or SD (19%), as opposed to achieving a CR at the time of initial scan15. Of the fifty-nine patients who achieved a CR on I+N, 19 (32.2%) remained on therapy for 4 years. 94.7% (18/19) of patients who were continued on I+N had an ongoing response at the time of analysis. 45.8% (27) patients treated with I+N discontinued therapy and did not require additional treatment. 92.5% (25/27) of patients on I+N who discontinued therapy after a CR, had an ongoing response off treatment. This is in stark contrast to only 2.6% (14) patients who achieved a CR on S. Of the 14 patients with a CR, 3 remained on therapy, 3 had treatment discontinued and an additional 8 were started on subsequent therapy with 3 (100%), 2 (66%) and 7(87.5%) patients demonstrating an ongoing response4,14.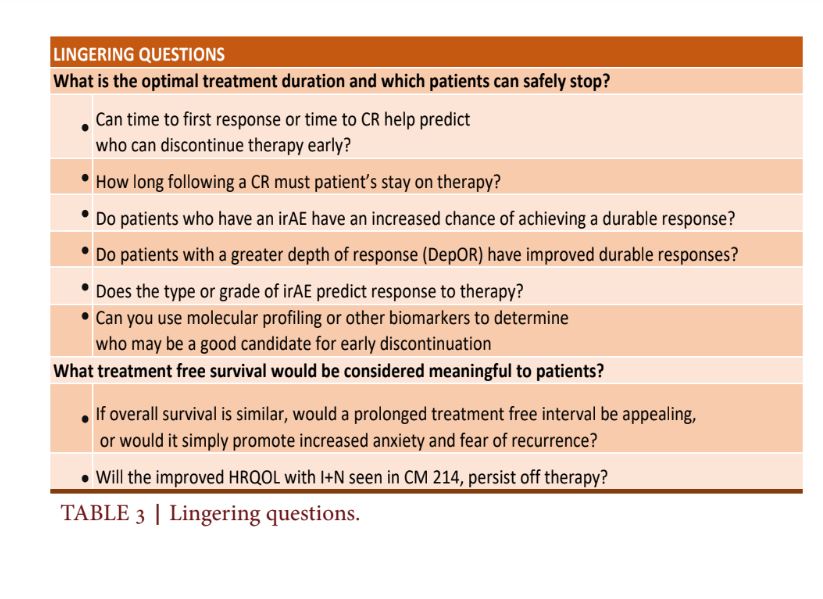
The percentage of patients with the ongoing response on I+N was almost identical for patients who continued on therapy compared to those who discontinued, 94.7% and 92.5%, respectively. Notably, only 21.4% (3/14) patients on S who had a CR discontinued therapy with 66% (2/3) of patients demonstrating an ongoing response. 22% (13) of patients treated with I+N went on to subsequent therapy after ICI discontinuation, though only 23% (3/13) of these patients had findings of progressive disease. Strikingly, in the favorable risk group treated with I+N, thirteen patients discontinued treatment with 5/13 receiving subsequent therapy despite only 7.6% (1/13) of patients demonstrating evidence of disease progression. Conversely, even after achieving a CR, 57% (8/13) of patients treated with S were started on subsequent therapy with 87.5% (7/8) with an ongoing response (Table 1)4. Based on these data, it may be reasonable to conclude that patients who achieve a CR on I+N can safely discontinue therapy with a high likelihood of having a durable response. Discontinuation may be further supported in patients with favorable risk disease who achieve a CR, though notably, combined immunotherapy is not approved in this setting. Further analysis of this group should include time to first response, time to complete response and time on therapy before discontinuation. Evaluation of minimal residual disease (MRD), circulating tumor DNA (ctDNA) and other pathologic factors should be investigated further, to help clinicians make educated treatment decisions.
TFS with Partial Response (PR)
One-hundred and fifty-six (28.5%) patients achieved a partial response on I+N. 17.9% (28) remained on treatment for 4 years, with 78.5% (22) of these patients demonstrating an ongoing response. In contrast, only 5.5% (9) of patients on S remained on treatment at 4 years, with 88% (8/9) maintaining an ongoing response. 42.9% (67) of patients treated with I+N discontinued therapy without the need for subsequent treatment. 31.3% (21/67) of patients with a PR off I+N eventually progressed, whereas 51.2% (20/39) of patients who discontinued therapy with S eventually progressed (Table 2)4. So, in summary, the CheckMate 214 data suggests there is about a 31% chance of disease progression for patients who discontinue I+N after a PR vs a 51% chance of disease progression in patients treated with S. This is in comparison to a 21% chance of disease progression in those who remain on therapy after PR compared to 11% of disease progression on S. These odds may give physicians pause when considering therapy discontinuation in patients with a PR. In the future, the Depth of Response (DepOR) should be further evaluated to see if patients who achieve a greater DepOR have improved durable responses after treatment discontinuation. Depth of Response in Contemporary Studies In the analysis above, patients who achieved a PR were not further separated by their Depth of Response (DepOR). Suarez et al looked at the association between DepOR and clinical outcomes in patients with advanced RCC, treated on CheckMate 9ER. This phase III trial compared cabozantinib plus nivolumab versus sunitinib in patients with advanced, previously untreated RCC. The depth of response was defined as the best percent tumor reduction from baseline. This study concluded that deeper responses led to improved 12-month PFS and 18-month OS rates. Interestingly, patients with a CR and PR1 (≥80% reduction in tumor burden) achieved similar OS18. The median time to respond was similar across groups, suggesting that time to respond may not be as vital. Further analysis should be pursued, to see if patients with varying DepOR can discontinue therapy early.TFS for Dual ICI Therapy in Context
Based on what we learned from CheckMate 214, when treated with dual checkpoint inhibitors, the TFS appears to be far longer than with targeted therapy alone. Tzeng et al sought to expand this data, performing a systematic review and meta-analysis to evaluate the treatment-free survival in objective responders with mRCC who discontinued ICIs13. Sixteen cohorts were analyzed, comprising 1833 patients treated with either ICI monotherapy, dual ICI or an ICI plus targeted therapy. A total of 572 (31.2%) patients had either a partial or complete response and 327 (57%) of those patients discontinued therapy. Interestingly, 85 (26%) patients demonstrated an ongoing response off therapy with TFS of 35%(95% CI 20-50%) and 20% (95% CI 8 to 35%) at 6 and 12 months respectively. However, these 16 studies were extremely heterogeneous. Differences in TFS between patients achieving a CR vs PR were not analyzed. When this data was broken down by treatment, the TFS was significantly higher when treated with dual immunotherapy as compared to an immunotherapy plus VEGF combination. Six- and 12-month TFS rates were 57% (95% CI, 41-73%) and 50% (95% CI, 32- 68) when treated with dual ICI as compared to only 20% (95% CI, 2-45%) and 5% (95% CI 0-17%) when treated with and an ICI plus VEGR TKI combination13. This significant difference should be considered when choosing initial therapy for patients whose goal is to achieve a period of TFS.Correlation of Immune-Related Adverse Events and TFS
The durability of response and treatment-free survival is especially important for patients who have had severe immune-related adverse events (irAE), as these are often a driving factor for treatment discontinuation. While some patients have mild irAE and can be restarted on therapy, others develop life-threatening issues mandating that treatment be halted. Treatmentrelated adverse events leading to discontinuation were more common with dual checkpoint inhibitor therapy vs sunitinib, occurring in 22% vs 13% respectively. Patients treated with ICI spent more time off treatment, with two-thirds of this time without a grade ≥2 treatmentrelated adverse events (TRAE). Conversely, patients treated with sunitinib had a shorter treatmentfree survival with about two-thirds of this time with a grade ≥ 2 TRAE12.Understanding the depth and durability of response, as well as the safety of restarting therapy following an irAE, is of utmost importance. A multicenter retrospective review by Alaiwi et. al. evaluated patients with mRCC who required at least a 1-week break on immunotherapy. Sixteen percent (80 patients) of patients required treatment interruptions with 45% able to restart therapy, while 55% percent discontinued treatment permanently. The median treatment break was 0.9 months (0.2-31.6 months). Following retreatment, half experienced a second irAE. Interestingly, only onethird of these patients experienced the same adverse reaction while two-thirds experienced a new side effect with the the median time to recurrent irAE of 2.8 months, which was similar to the time of first irAE, 2.7 months19. Future studies should investigate if patients who have an irAE have an increased chance of achieving a durable response. This should be further broken down by degree and type of irAE.
Dosing Strategies to Promote TFS with IO Therapy
While prolonged TFS has the potential to improve QOL, alternative dosing strategies may also improve toxicity profiles and patient-reported outcomes, without reducing OS. Intermittent dosing strategies have been under investigation to help answer these questions. Ornstein et al conducted a small phase II trial to evaluate the role of intermittent nivolumab dosing for patients with IMDC I/P risk mRCC, previously treated with antiangiogenic therapy with the hope of gaining additional insights into optimal treatment schedule and duration. Patients were treated with nivolumab monotherapy for twelve weeks at which point disease response was assessed. Patients with less than 10% tumor burden reduction were continued on nivolumab monotherapy and reassessed at 3-month intervals. However, if patients had ≥10% tumor burden reduction they were placed in a treatment-free observation phase, again with imaging every 3 months. This classification and intervention were continued until the RECISTdefined progression of disease (PD). Patients who did not achieve at least 10% tumor burden reduction at 6 months were removed from the study and treated with nivolumab standard of care 10.Fourteen patients were included in the study. ORR was 29%, with 4 patients (29%) achieving a PR, 6 with SD (49%) and 4 with PD (29%) at a median follow-up of 6 months. Median PFS was 7.97 months. Five out of fourteen (38%) of patients were eligible to stop therapy and all agreed. Four out of the five patients achieved this response after only 12 weeks of treatment. At median follow up of 48 weeks only 1 patient needed to restart therapy. The four remaining patients have had a clinical response for a median of 34 weeks (range 16- 54) off therapy and a median tumor burden decrease of 46.5% (38- 80%)10. This study demonstrates that patients may be interested in less frequent therapy, with the notion that treatment breaks result in decreased cumulative toxicity, with the possibility for decreased adverse events along with possibly reduced financial toxicity.
CONCLUSION AND SUMMARY
Systemic treatment of metastatic renal cell carcinoma has dramatically improved in the last 5 years with the use of immune checkpoint inhibitors and targeted therapy. These treatment breakthroughs have led to improved overall survival, though currently, treatment for mRCC remains indefinite. When it comes to first-line treatment, physicians have a variety of therapeutics to choose from including dual ICI and ICI/ TT combinations. There are many patient and disease-specific factors that can help guide these important treatment decisions. For example, a critically ill patient with the need for a rapid treatment response would likely choose the ICI+TT combination over dual ICI. Similarly, a patient with a strong history of autoimmune disorders may choose to forego ICI therapy altogether and begin TT monotherapy. However, for the right patient, dual ICI therapy offers the potential for a more durable response with improved TFS and QOL8, 12, 13. Overall survival is the hallmark of effective treatment. However, many other factors should be considered when determining the ideal treatment strategy, including treatment tolerability, risk of TRAE, the durability of response, and the ability to discontinue therapy in favor of close monitoring. CheckMate 214 and subsequent subanalyses provided great insight into answering these questions. Patients treated with dual ICI were noted to have more durable and deeper responses, as compared to ICI/TT combination or TT monotherapy12, 13. Overall, TFS was more than two times longer with dual ICI vs S, 6.9 months compared to 3.1 months which represents meaningful time away from the hospital and clinic12. Patients treated with dual ICI also had a significantly higher chance of achieving a CR, 10.7% as opposed to only 2.6% of patients treated with S. In this subset of patients, the mean TFS after dual ICI was 34.6 months (0.5-49.7) with objective responders treated for an average of 20.6 months (17.7-23.2) before treatment discontinuation14,15 Secondary analyses noted that patients with lower disease burden and higher PD-L1 status were more likely to achieve this coveted CR, though more robust data is needed to help predict who will best respond to therapy15.Ultimately, there are many questions still left unanswered. The optimal treatment duration for patients treated with dual ICI is still unknown. After considering that the data presented above, it may be reasonable to consider treatment discontinuation for patients who achieved a CR, in favor of active surveillance. Ongoing response rates after CR was similar regardless of whether therapy was continued or stopped, 94.7% versus 92.5% respectively. However, based on the data currently available, the risk of discontinuing therapy after a PR in the I/P risk population may be too great. Future studies should further evaluate TFS based on the depth of response (DepOR), as patients with a deeper DepOR may also have a prolonged TFS, similar to those with a CR. Additionally, biomarkers, such as the use of next-generation sequencing results, ctDNA and MRD, may prove beneficial to help predict who may best respond to ICI therapy. For example, patients with more favorable mutations, such as PBRM1, may be able to discontinue therapy earlier than patients with BAP1 alterations, which have been traditionally associated with a worse prognosis20. Finally, while CheckMate 214 reported improved patient-reported outcomes on dual ICI vs S, there is limited QOL data during the treatment-free period. These and other questions must be answered to provide patients with improved treatment strategies and QOL, Table 3.
REFERENCE