Submitted - December 14, 2021 | Accepted - January 10, 2022 | | ePublished - March 17, 2022
https://doi.org/10.52733/KCJ20n1-a1
The Latinx Disparity in Surgery for Kidney Cancer: Data from The South Texas Region
Furkan Dursun, MD,1 Rahul S. Patel, MS,1 Dawn S. Hui, MD,2 Hanzhang Wang, MD,1 Ahmed M. Mansour MD,1,3, Deepak K. Pruthi, MD,1,3, David G. Alonzo, MD4, Lalithapriya Jayakumar, MD5, Ronald Rodriguez, MD1,3, Robert S. Svatek, MD1,3, Michael A. Liss, MD1,3, and Dharam Kaushik, MD1,31. Department of Urology, University of Texas Health San Antonio, TX, USA
2. Department of Cardiothoracic Surgery, University of Texas Health San Antonio, TX, USA
3. University of Texas Health San Antonio/ MD Anderson Mays Cancer Center, TX, USA
4. Department of Urology, University of Texas Rio Grande Valley, TX, USA
5. Department of Surgery, Division of Vascular & Endovascular Surgery, University of Texas Health San Antonio, TX, USA
ABSTRACT
South Texas region, with a predominantly Latinx population, has a very high incidence of renal cell carcinoma (RCC), including those with tumor extending into the major blood vessels called venous tumor thrombus (VTT). There is currently no data on outcomes of Latinx patients with VTT as most published studies are from predominantly Caucasian population. Therefore, we performed this study to fill an urgent, unmet need. We reviewed patients who underwent radical nephrectomy with removal of VTT (called tumor thrombectomy) between 2015 and 2020. We collected data on demographics, clinical, pathological characteristics and outcomes of patients. Univariate and multivariate Cox regression analyses were used to evaluate the associations between ethnicity and disease progression or survival. We identified 112 patients, of which 67 (62%) were Latinx, and 41 (38%) were non-Latinx. Approximately 60% of patients had Level II-IV VTT; Latinx presented with a higher level of tumor thrombus (p=0.046). Latinx patients had a higher rate of no insurance (11% vs. 27%, p=0.04) and were more likely being lost to follow-up after surgery (22.4% vs. 13.3%, p=0.23) compared to non-Latinx. Fewer Latinx received systemic therapy (28% vs. 42%; p=0.13). Ninety-day mortality for the entire cohort was 3.8%. The Latinx population in the South Texas region present late, with advanced thrombus level, and do not have access to systemic therapy. Given symptomatic disease, surgical treatment, if feasible, is their only option. Our results highlight disparate treatment patterns which require further investigation and health-care policy changes.
INTRODUCTION
Kidney cancer is the 8th most common malignancy in the United States with an estimated 73,750 new cases and approximately 15,000 mortalities in 2020.1 Renal cell carcinoma (RCC) has a tendency for vascular invasion: 10- 15% of patients have venous tumor thrombus (VTT) and 1-3% of cases have tumor thrombus extending into the right atrium. 2,3 Inferior vena cava involvement or obstruction by tumor thrombus can produce various clinical manifestations, including gross hematuria, lower extremity edema, varicocele, abdominal distension with hepatic dysfunction (possibly related to a Budd-Chiari syndrome), fatigue, malabsorption, shortness of breath, and pulmonary emboli. 4
Even in the era of targeted therapy and subsequent immune checkpoint inhibitors, radical nephrectomy (RN) with tumor thrombectomy remains the mainstay of treatment for RCC patients with VTT. This aggressive surgery has significant morbidity and mortality, but the 5-year survival rates can be significant as up to 70%, especially for patients without metastatic disease. 5
Contemporary management of RCC with VTT requires an interdisciplinary approach and resources to provide the best curative to palliative care, which is mostly available in tertiary care centers. Unfortunately, racial disparities exist both in the access to health care and the quality of care administered in the U.S.6,7 Most of racial disparity studies have focused on differences between African Americans and non-Latinx Whites (NLW), there is paucity of data in RCC regarding disparities in care among the Latinx population, which is the largest minority with 18.5% of the total U.S. population. 8 With a predominantly Latinx population, the South Texas region has a very high incidence of RCC, including those with VTT. 9 Of all the South Texas Latinx, 31-61% are uninsured.10 This disparity results in delayed presentation of patients with RCC. However, there is a paucity of data on outcomes of Latinx patients who underwent extirpative surgery for their symptomatic disease. Since most published literature on tumor thrombus patients is in the predominantly Caucasian population with insurance, we performed this study to evaluate disparities between Latinx and non-Latinx with RCC and VTT in South Texas region. Our institution is a tertiary referral center with a catchment area of 2.5 million. We are uniquely positioned as we accept all South Texas patients for surgical services, regardless of their insurance status.
METHODS
Patient Selection
After approval by the Institutional Research Ethics Board (IRB number: HSC2021108E), we reviewed the medical records of our prospectively maintained database of patients who underwent RN with tumor thrombectomy for RCC between 2015 and 2020 at our institution.
Covariates
We abstracted patient-level characteristics including age at diagnosis, sex, ethnicity, body mass index (BMI), American Society of Anesthesiologists (ASA) classification, smoking status, insurance status, and comorbidities such as diabetes mellitus (DM), hypertension and hypercholesterolemia. Socioeconomic variables including annual household income, education level and distance from home to hospital were estimated from county of residence by using patients’ self-reported zip code. Ethnicity and race were determined by patient self-report on their electronic medical records (EPIC). Disease characteristics included clinical TNM stage, 11 thrombus level, maximum clinical tumor size and preoperative lab values. Mayo Clinic RCC tumor thrombus level classification system was used for thrombus level. 12
Operative data included total surgical time, surgical approach and incision type, type of bypass, use of intraoperative transesophageal echocardiography (TEE), utilization of liver mobilization or Pringle maneuver, resection of IVC, estimated blood loss (EBL), blood transfusion during surgery, and perioperative complications. Pathological characteristics of the tumor were collected from the pathology report. Early postoperative complications (< 90 days) were classified according to the Clavien-Dindo classification system. 30-day readmission rates, 90-day mortality rate, patient follow-up data including local recurrence, disease progression, and survival, as well as receipt of neoadjuvant or adjuvant chemotherapy, were recorded. Progression of disease was classified as attenuated (not until at least 6 months after surgery) or rapid (progression or death within 6 months of surgery).
Statistical Analysis
The associations between ethnicity (Latinx or Non-Latinx) and demographic or clinical characteristics were assessed with Fisher’s exact test and the two-sample Wilcoxon rank-sum test for continuous variables. Chi square test was used for assessing categorical variables. Univariate and multivariate Cox regression models were used to evaluate the associations between ethnicity and disease progression or survival. Significance was set at 0.05 and all p values were two-tailed. Statistics was performed using STATA 15.0 (StataCorp, College Station, Texas).
RESULTS
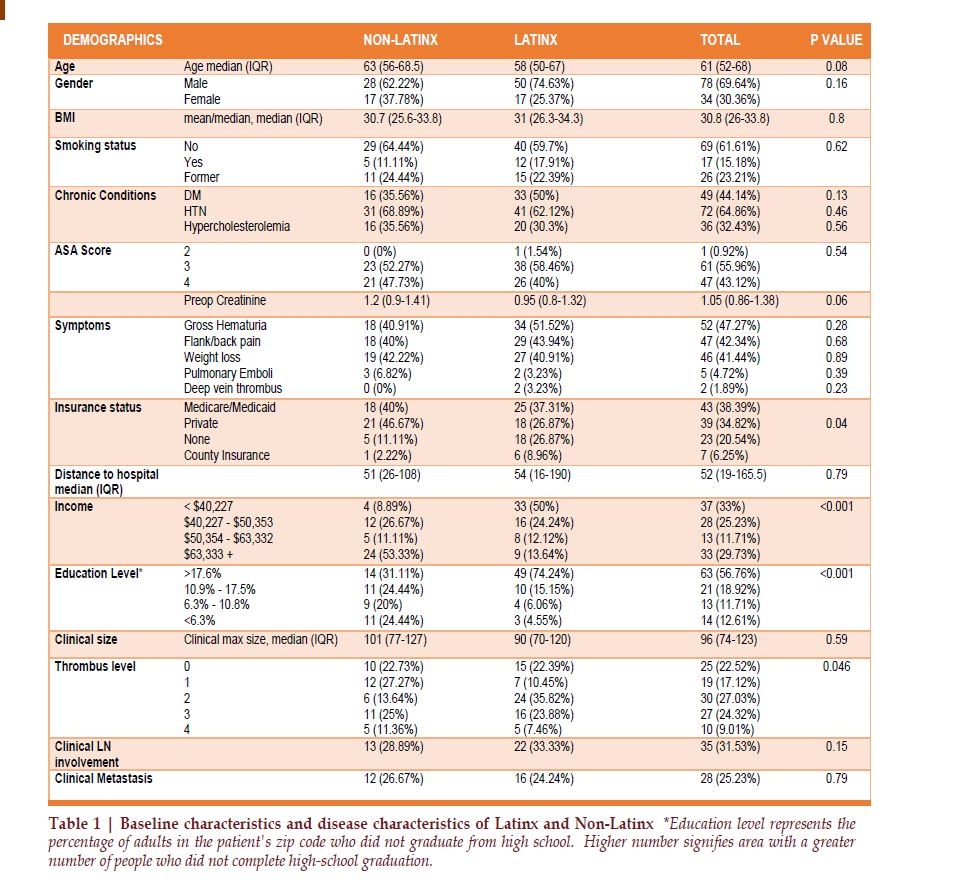
We identified a total of 112 patients with bulky advanced RCC and VTT who underwent RN and tumor thrombectomy, including 67 (60%) Latinx and 45 (40%) non-Latinx patients. There were no significant differences observed between Latinxs and non-Latinx across age, sex, accompanying chronic conditions, ASA classification and BMI (Table 1). Most of the patients were male (70%) and more than half were obese with a median BMI of 30.8 (IQR 26-33.8). All but one patient was classified as ASA III (56%) or IV (43%). They had accompanying chronic conditions such as diabetes mellitus (44%), hypertension (65%), and hypercholesterolemia (32%). 15% of patients were active smokers. More than three-quarters (78%) of the patients had at least one complaint of constitutional symptoms; weight loss (41%), gross hematuria (47%), and/or flank pain (42%). Latinx patients had significantly higher rate of no insurance compared to non-Latinx (26.9% vs. 11.1%, p=0.04). There was no significant difference for distance from home to hospital between Latinx and non-Latinx (54 miles Vs. 51 miles, p=0.79). The Latinx population had significantly lower annual income (p<0.001) and rate of high school graduation (p<0.001).
Clinical and Perioperative Features
The median clinical maximum tumor length at presentation was 96 mm (IQR 74-123). Thirty two percent of the patients presented with clinical node positive disease and 25 % with metastatic disease. Sixty-seven (60%) patients had level II-IV tumor thrombus indicating advanced disease. Sixty-two (55%) patients had right-sided tumors, which were more likely to present with level III or IV tumor thrombus than leftsided tumors (40% vs. 24%; p=0.07). Latinx presented with higher level of tumor thrombus (p=0.046). Level II-IV thrombus was seen in 67% of Latinx vs 50% of non-Latinx (Table 1).
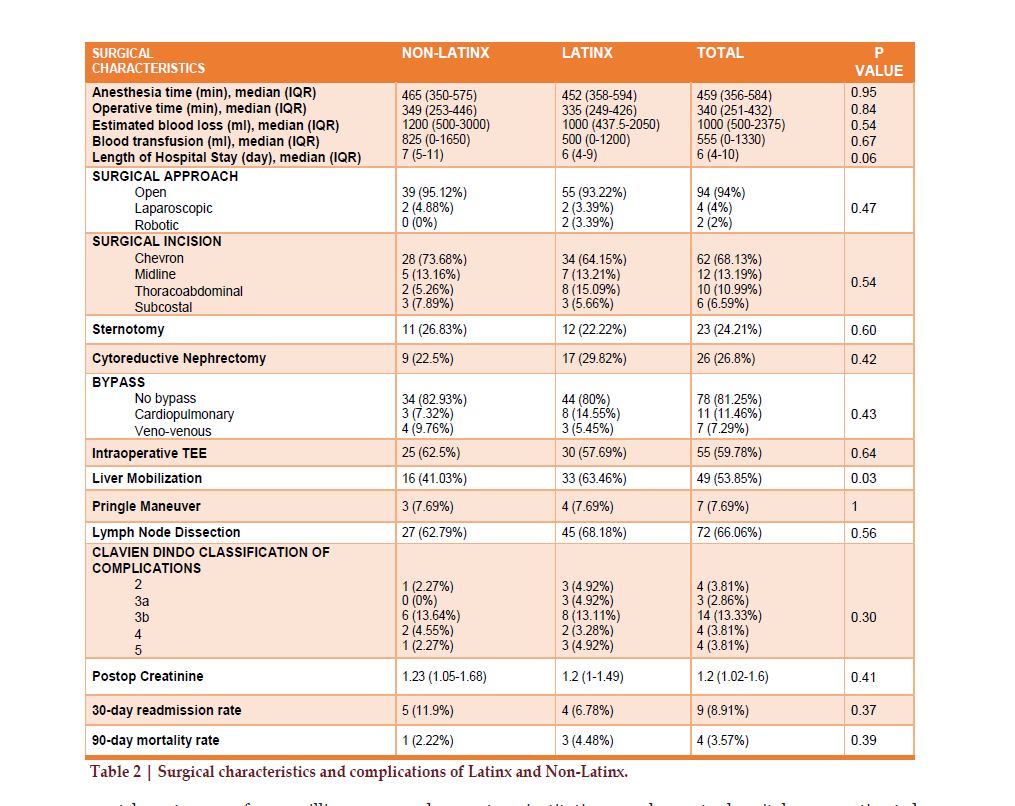
The median operation time was 340 minutes (IQR 251-432). The median EBL was 1000 ml (IQR 500-2375) and the median blood transfusion during the surgery was 555ml (IQR 0-1330). There were significant increases in the EBL during surgery as the tumor thrombus level increased (p=0.003). There was also an increasing trend in the amount of blood given during surgery as the tumor thrombus level increased(p=0.08). Table 2 lists the differences observed between Latinx and non-Latinx across intraoperative features, perioperative complications, 30-day readmission date and 90-day mortality. Latinx patients, due to the high rate of level II-IV tumor thrombus, indicating advanced disease burden, were more likely to have a thoracoabdominal incision compared to non-Latinx (15% vs. 5% p=0.21). Also, Latinx underwent more complex surgical maneuvers such as complete hepatic mobilization during surgery than non-Latinx (63% vs. 41%, p=0.03). The radical nephrectomy was done for cytoreductive purposes for 27% of the patients, and retroperitoneal lymph node dissection was utilized for 66% of the patients. Given advanced disease states, 60% had intraoperative TEE to evaluate in real time the level of thrombus during the surgery.
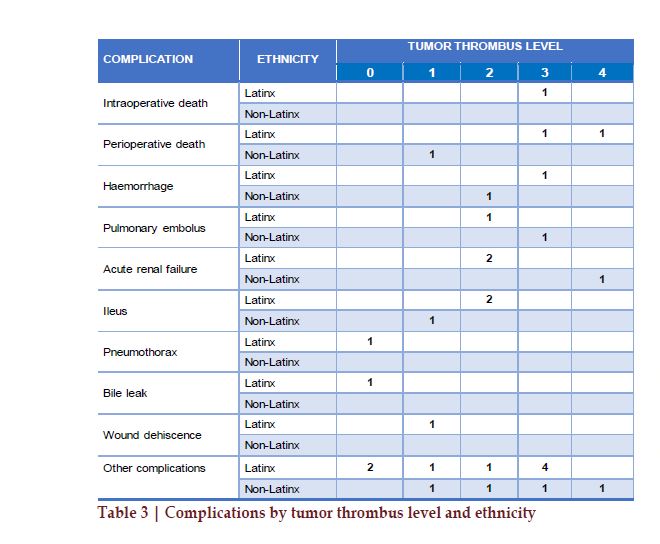
The 90-day surgical complications are summarized by tumor thrombus level and ethnicity in Table 3. One patient died during the surgery and three patients died in the postoperative 90 days (2.7%), with a total perioperative mortality of 3.6%. The median length of hospital stay was six (IQR 4-10) days. There was no statistically significant difference in the duration of hospitalization by tumor thrombus level (p=0.11) or by ethnicity (p=0.06). 30-day readmission rate was 9% and there was no statistically significant difference in the readmission rate by thrombus level (p=0.56) or by ethnicity (p=0.37).
Tumor Histopathology and Survival Outcomes
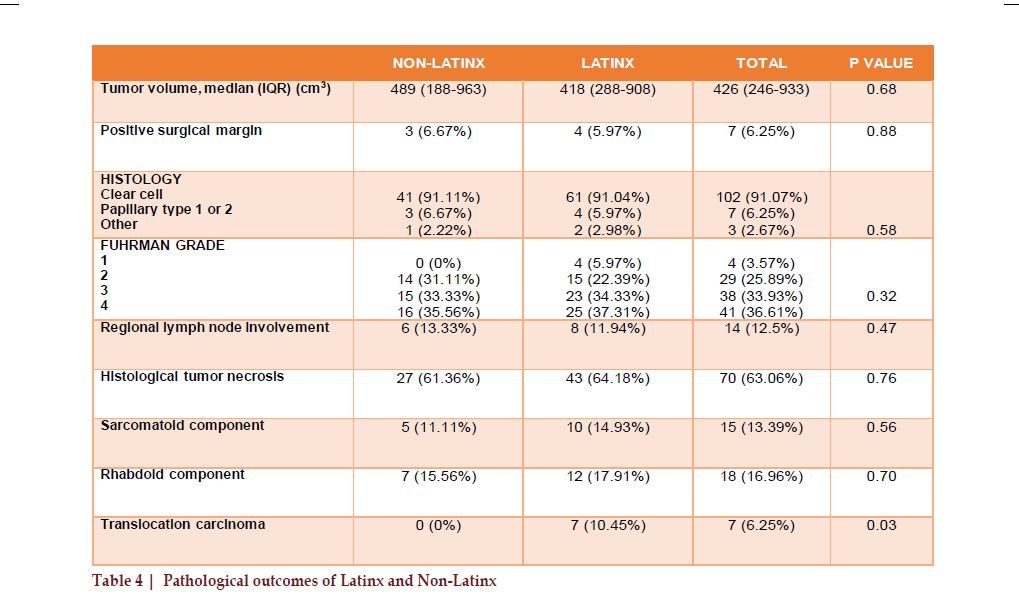
The median tumor volume was 426 cm3 (IQR 246-933) on the pathological evaluation. Fourteen (12.5%) patients had regional lymph node involvement, and seven patients (6.3%) had a positive surgical margin. Among 112 patients evaluated, 102 (91.1%) had clear cell RCC, and 7 (6.3%) had papillary RCC. There was no significant difference in the tumor histology by ethnicity (p=0.58). Pathologic features are listed in Table 4. More than 70% of the patients were marked as grade 3 or 4 within the Fuhrman grading system- an indicator of very aggressive cancer. Additionally, 63.1% of the patients had histological tumor necrosis, 13.4% had sarcomatoid differentiation, 17% had rhabdoid differentiation, and 6.3% had translocation carcinoma. Latinx patients had higher incidence of very aggressive variant of RCC called translocation carcinoma: seven patients were reported with translocation renal cell carcinoma, and all of them were Latinx (p=0.03).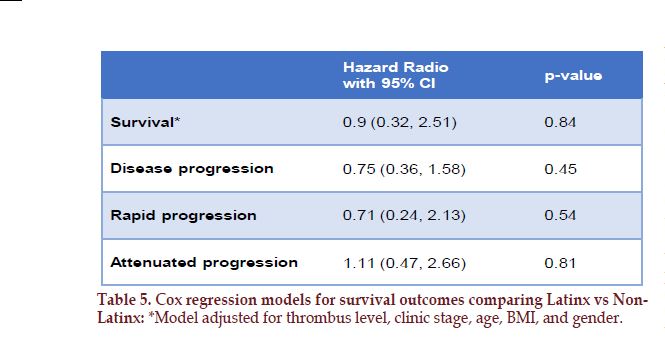
The median follow-up time was 453 days (IQR 129-1125). The Latinx was more likely had no postoperative follow-up compare to non-Latinx. (22.4% vs. 13.3%, p=0.23). There were no significant differences in 2-year and 3-year survival for Latinx and Non-Latinx patients (82% vs 83%, and 75% vs 77%, respectively, p=0.81 and 0.98). Forty-eight patients (43%) had progression, and twenty-one (18.8%) patients died of advanced RCC. Patients with higher Fuhrman grade were more likely to have disease progression (HR=2.08 p=0.001). Of those patients with metastatic disease, 33% received systemic therapy. We observed less utilization of systemic therapy in the Latinx group compared to the non Latinx group (28% vs. 42%; p=0.13). There were no statistically significant differences in 2- and 3-year survival rates when compared patients with level 0-II tumor thrombus and patients with level III-IV tumor thrombus (2- year survival: 83% - 79% p= 0.687, and 3-year survival: 76% - 74% p=0.454, respectively). In the multivariate cox regression model adjusted for thrombus level, clinic stage, age, BMI, gender; the ethnicity was not found to be a significant prognostic factor for disease progression and survival (HR 0.75, CI 0.36–1.58, p= 0.45 and HR 0.9, CI 0.32–2.51, p= 0.84 respectively) (Table 5).
DISCUSSION
Texas is home to approximately 19% of Latinx population in the US, majority of which are concentrated in the South Texas region. Since kidney cancer is the 4th leading cause of death among Latinx males, we attempted to study the disparity and variation in a highly complex presentation- kidney cancer with tumor thrombus. The idea behind performing this study was to understand disparities to make system-based changes. Our goal was to twofold: to identify the disparity in presentation of advanced kidney cancer and identify differences in quality of care exist between Latinx and non-Latinx undergoing radical nephrectomy with tumor thrombectomy. In addition, we sought to share our experience with this challenging, high-risk procedure in a predominantly Latinx cohort. In this high volume, single-institution, retrospective study, we found that the Latinx in the South Texas region presented late, with advanced thrombus level (67% vs. 50%, p=0.046). The uninsurance rate was significantly higher in Latinx than non-Latinx (11% vs. 27% p=0.04), impacting the number of patients lost to followup and receiving systemic therapy. Our results highlight disparate treatment patterns across patients. Despite delayed presentation, our study shows that rapid coordination of extirpative surgery enhances the outcome for these patients with equivalent perioperative outcomes.Latinx is the largest and youngest racial/ethnic minority group in the US and rapidly increasing in population size which is expected to be doubled over the next four decades.1 Unlike non- Latinx, cancer is the leading cause of death among Latinx, even though the cancer incidence is not higher than non-Latinx.14 Latinx are more likely to present with an advanced disease which partly contributes to higher mortality rates, especially for colorectal, endometrial, liver, and stomach cancers.15 The incidence and mortality of kidney cancer for Latinx living in the US is similar to all US populations.1 But the incidence of kidney cancer in Texas, especially in South Texas, is higher than in the U.S., with Latinx significantly having the highest incidence.9 Two recent population-based studies reported data on racial disparities in RCC. Callahan et al. assessed the reasons for the incidence disparity of RCC in African Americans and Whites, but Latinx were not evaluated in this study.16 They reported that interventions that prevent hypertension and chronic kidney disease among African Americans could potentially eliminate the racial disparity in RCC incidence.16 Utilizing the SEER database, Whitson et al. evaluated the prognostic factors of RCC patients with VTT, and they reported race was not associated with mortality (HR 1.2 CI 0.5–2.9, p=0.63).17 Race or ethnicity was not reported in the prior case series for RCC and VTT. 18-21 Our study is unique, evaluating the disparities in patient presentation, treatment approaches, and outcomes for Latinx and Non- Latinx patients with RCC and VTT. It provides a contemporary real world scenario from South Texas- a region with very high incidence of kidney cancer, low insurance, diverse cultural and ethnic background.
RN with tumor
thrombectomy has proven long-term
disease-free survival, especially for
patients with the non-metastatic
disease.22, 23, 18, 20 The clear cell
carcinoma is the predominant
histology reported, changing
between 70 to 93% in different case
series.19, 20, 21, 24 Neither thrombus
level nor histological type was
associated with progression and
survival.18-24 Likewise, most of our
cases were clear cell RCCs (91%) and
the histologic type and thrombus
level were not associated with
disease progression or survival.
Surgical management of RCC with VTT is challenging and requires multidisciplinary perioperative care. The surgical approach may involve full liver mobilization and thoracotomy to allow access to the intrahepatic and intrapericardial tumor thrombus especially for patients with level II-IV VTT. The intraoperative and postoperative death in 30 or 90 days was reported between 2.9 - 6.1% in the largest series.18-21, 25 In the same series with available data, the perioperative death rate for level III-IV tumor thrombus patients was reported between 5.6-14.6%.18- 20, 24 Similar to those studies, the perioperative death rates of our cohort and for the subgroup of patients with level III-IV tumor thrombus was 3.6% and 8.1%, respectively.
Xp11.2 translocation RCC is caused by increased expression of transcription factor for immunoglobulin heavy chain enhancer 3 (TFE3) as a result of translocation of the TFE3 gene on chromosome Xp11.2. It mainly occurs in children and young adults, accounting for 20-40% of RCC cases in children. The incidence is low in adults accounting for approximately 1% of RCC and there are only 7 cases reported over the age of 65 years.26,27 The prognosis is less aggressive in children and adolescents, but shows poor prognosis in adults.28 Because of the rarity, our understanding of its pathogenesis is incomplete and there is no data about the ethnic differences in the incidence of Xp11.2 translocation RCC. We reported 7 patients with translocation carcinoma and all of them were Latinx (p=0.03) with a median age of 50 years (range 21 to 67). Latinx patients had higher incidence of this aggressive variant of RCC which needs further investigation.
This study has several limitations. Foremost, this retrospective single-institution study is limited by inherent biases in patient selection, data collection, and analysis. The utilization of preoperative embolization, lymph node dissection, bypass, and surgical approach was based on the surgeon's clinical judgment and experience which may be a source of additional bias. The generalizability of our results is limited due to the high Latinx population in the South Texas region. However, the fundamental strength of this study lies in the analysis of a large sample size. To our knowledge, this is the first study assessing disparities in the presentation and quality of care between Latinx and non-Latinx RCC patients with venous tumor thrombus.
In conclusion, compared to Non-Latinx, the Latinx population in the South Texas region presents late, with advanced thrombus level, and does not have access to systemic therapy. Surgical treatment, if feasible, is their only option. Our results highlight disparate treatment patterns. A better understanding of the factors that cause such a high rate of uninsurance in the Latinx is crucial to creating more appropriate and effective reform strategies.
FUNDING
NoneAUTHOR CONTRIBUTION
DK planned the concept of the study and DK, FD planned the study design. FD, RP, and DK collected and verified the data. HW analyzed the data and DK, FD, RR and HW were involved in data interpretation. FD and RP wrote the first version of the manuscript. FD, RP, DH, HW, AM, DP, DA, LJ, RR, RS, ML and DK contributed to interpretation of the results and revision of the manuscript and approved the final version of the manuscript. Project administration was conducted by DK.Declaration of competing interests
The authors have no conflicts of interest.ACKNOWLEDGEMENTS
Furkan Dursun has received research support through the University of Texas Health Science Center at San Antonio Cancer Research Training Program supported by the CPRIT Research Training Award (RTA; RP170345). Dharam Kaushik is supported by Stanley and Sandra Rosenberg Endowment in Urologic ResearchREFERENCES
Correspondence to: Dharam Kaushik M.D. Department of Urology, University of Texas Health, San Antonio, TX 78229-3900.
Email: kaushik@uthscsa.edu; drdkuro@gmail.com



