https://doi.org/10.52733/IKCSNA-Proc-or5

* # Lars Lund, M.D.
Department of Urology, Odense university Hospital, DK-5000 Odense, Email: Lars.Lund@rsyd.dk
INTRODUCTION
In Denmark, approximately 80 approved nationwide clinical quality registers support the Danish healthcare system with data analysis and reports to secure development and improvement of the clinical quality. These registers typically cover all patients with a specific disease or those undergoing a specific procedure and includes 27 clinical quality registries in cancer care and cancer screening united by the Danish Multidisciplinary Cancer Group1, 2 . The registers are operated by the Danish Healthcare Quality Institute (DHQI) (formerly the Regional Clinical Quality Development Program) 1, which contributes to the daily operation, analysis and reporting. To obtain approval and public funding, the program requires that a quality register covers at least 90% of the Danish patients with a disease3 . For each register, a professional steering group has been established to determine what the content should be – including the individual quality indicators and quality targets goals). DMCG.dk was founded in 2004 and is the association of Danish Multidisciplinary Cancer Groups, whose main task is to promote cancer treatment in Denmark4 . here are 25 DMCGs that focus on, among other things, supporting clinical quality registers and preparing clinical guidelines for diagnostics and treatment (Figure 1).
Clinical quality development and indicator sets in the clinical quality databases
The primary purpose of the clinical quality register is to monitior the quality of the healthcare systems efforts and results for a defined group of patients in order to contribute to healthcare quality improvements. Registrations in these registers are mandatory for all the relevant clinical departments and service providers. Reporting to the quality register can be done without patient consent5 . The first Danish clinical registers were established by enthusiastic clinicians who felt a need to get insights into the clinical profile of the patient population including the treatments they were offered and the clinical results6 . The current focus is on supporting the development and improvement of high and uniform quality of care in the treatment process across the country based on international understandings of the concept of quality7,8 . Quality can be viewed from a clinical, patient-experienced and organizational perspective. In the registers, the clinical perspective has been predominant, but work is done to ensure that the patient perspective will increase by i.e. including patients’ representatives in the steering group. The overall aim is to secure development and improvement of quality throughout the entire patient journey across the healthcare sectors (specialist practice, general practice, hospital, and municipality).
Figure 1. Danish Multidisciplinary Cancer Groups.
The clinical quality register allow for uniform national monitoring across the country. The target group and purpose of use are crucial for how frequently and how data should be analyzed and knowledge communicated. The overall thinking in the work is an improvement model with the following elements: a) Improvement goals b) Establish measurement (indicator) c) Select change (intervention) d) Test change e) Assessment (audit). The efforts are focused on creating national consensus on quality and challenges in the current disease area, and on measuring, assessing and reporting results.
Darenca is the DMCG for kidney cancer. The group consist of 5 urologists, 3 oncologists, 1 clinical epidemiologist, 2 pathologists, 2 diagnostic imaging specialists, 1 clinical geneticist and 1 patient representative. The Darenca group meet several times during the year to prepare an annual quality report, which is sent to all hospitals, hospital management, politicians and the Danish Health Authority. The annual report is finalized by DaRenCa members in collaboration with methodological experts from DHQI. The register DaRenCaData includs all persons with a first-time diagnosis of renal cancer in Denmark since August 20109 .
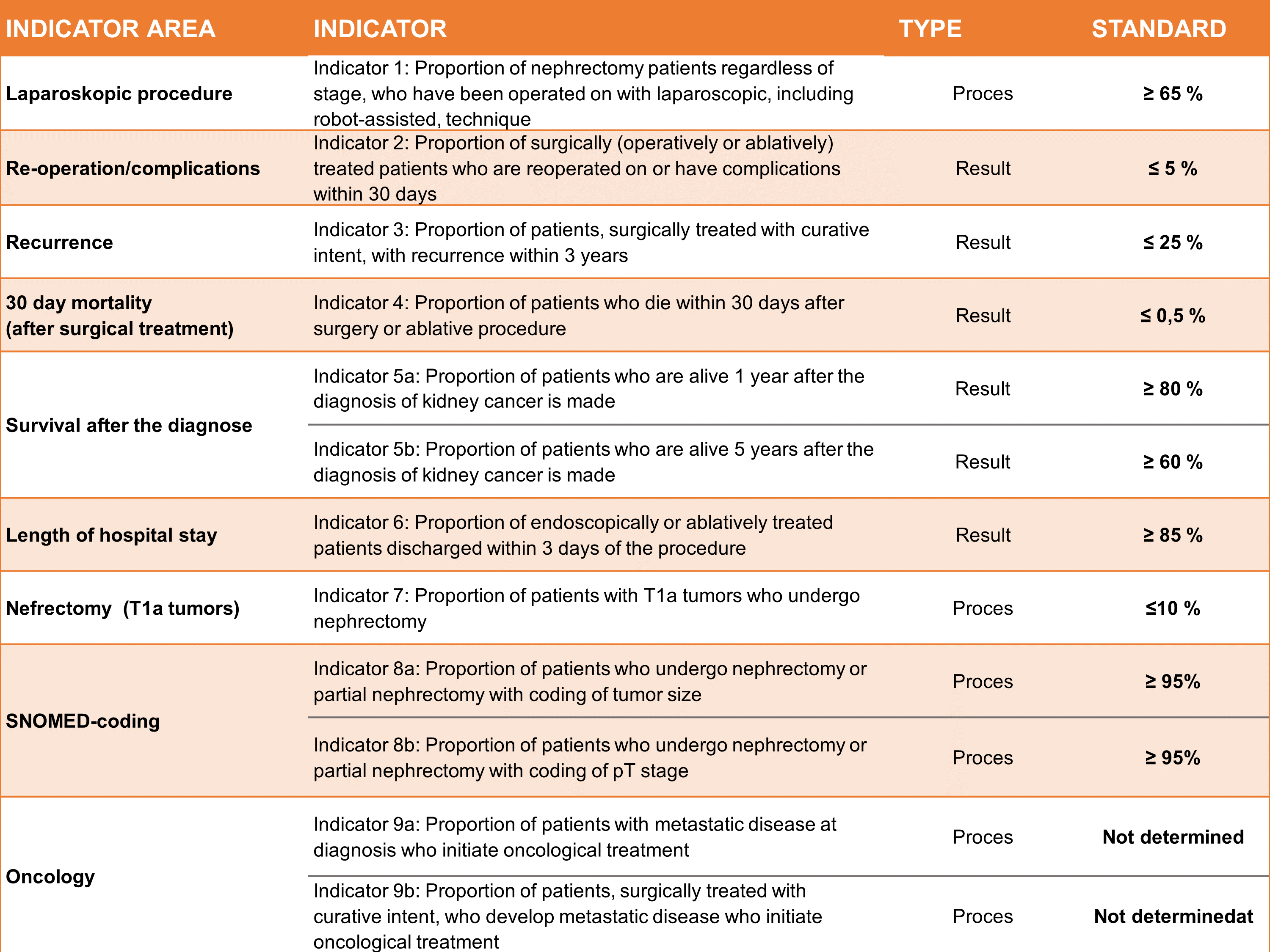
Table 1 . Characteristics of Indicator.
The annual DaRenCa quality report
The annual report includes two patient populations: A. Patients with newly diagnosed histologically or cytologically verified kidney cancer (Patient population 1) and B. All surgically treated patients with histologically verified kidney cancer (Patient population 2). Population 1 is based on pathological diagnoses recorded in the National Registry of Pathology (LRP), while Population 2 is based on data from both the Danish National Registry of Patients (DNRP) and the LRP.An overview of indicator set as published in 2024 is shown in table 1 and some results in table 2. At the end of 2023, the register included 12,193 new cases of kidney cancer in Denmark from its inception on 1 August 2010 to 31 July 2023, of which 1091 in the current year (the period 1 August 2022 to 31 July 2023). Only patients with histologically or cytologically verified kidney cancer are included in the register. This is unique to Denmark, as patients in other countries patients are also included if the diagnosis is based solely on imaging.
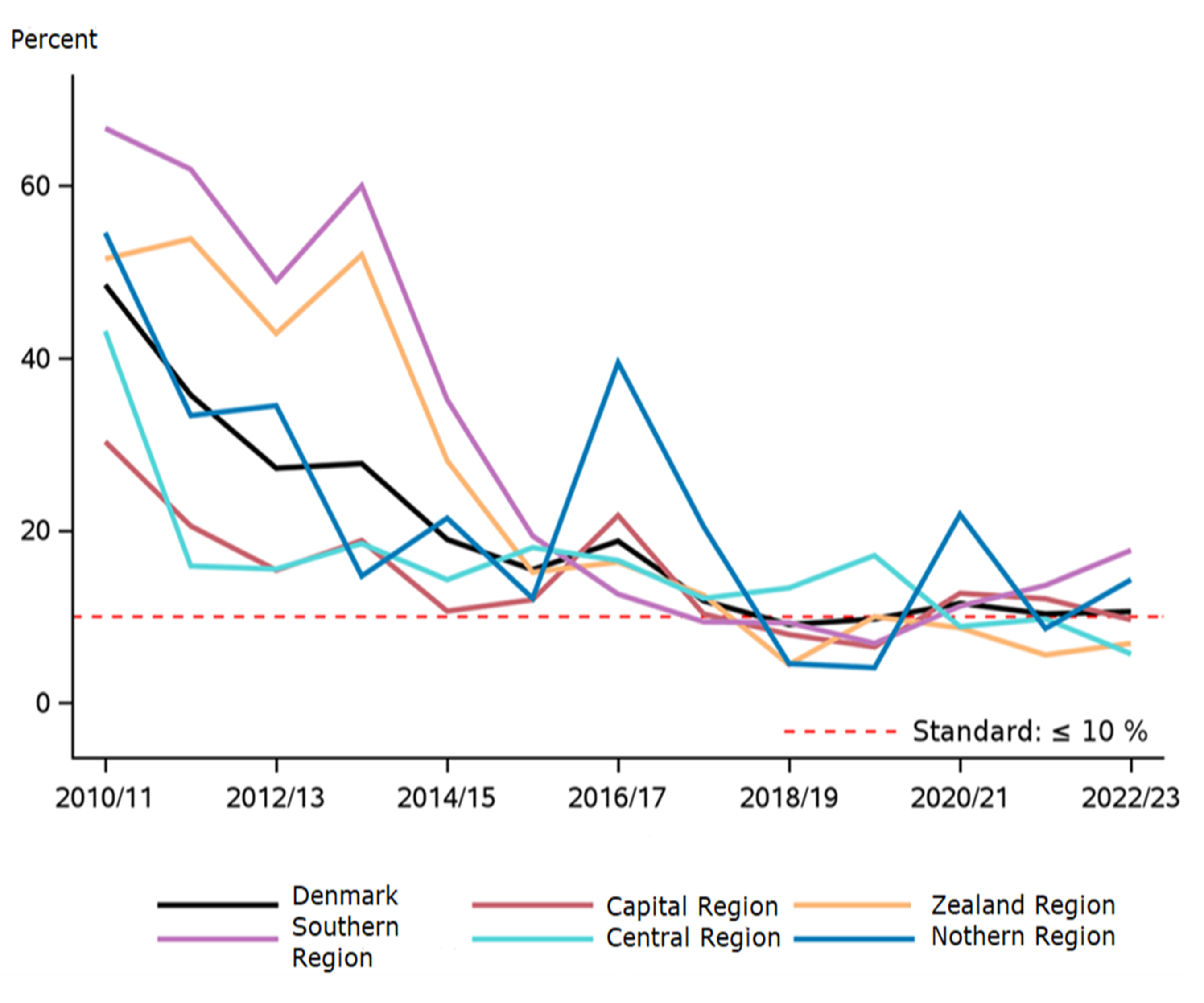
Figure 2. Proportion of nephrectomies over time for T1A tumors.
Incidence rates of kidney cancer have shown an increasing trend in most countries over the past 10 years (5), which is also the case in Denmark. In the 2023 report, the incidence rate was 17.3 per 100,000 inhabitants, and in 2022-23 it was 25.5 for men, increasing, and 10.3 per 100,000 for women, stable over time. The disease is most frequently diagnosed at the age of 60-70, with a median age of 69 years in the 2023 report and the distribution between men and women was constant, 69% and 31% respectively.
Survival after kidney cancer is increasing in Denmark. The observed 1-year survival was in 2023 90%, which indicated a slightly increasing trend compared to 89% and 88% in the 2 previous periods (Table 2). The observed 5-year survival was 68% compared to 70% and 65% in the 2 previous periods. The relative 5-year survival, which is the most often stated in international publications, was 75% for both men and women. In the period 1994-2003, the relative 5-year survival for kidney cancer in Denmark was 39% for men and 44% for women. Over the years, there has been a significant improvement in the survival of Danish kidney cancer patients, as an outcome of different activities including publications from the clinical register and national audits of the results, systematic work with national treatment guidelines, as well as introduction of new treatments. A contributing factor to the improved survival may be the increase in the proportion of patients diagnosed at an early stage of the disease. At the same time, improved treatment options for patients with locally advanced and metastatic disease have meant that survival is also increasing for patients with stage III or IV disease.
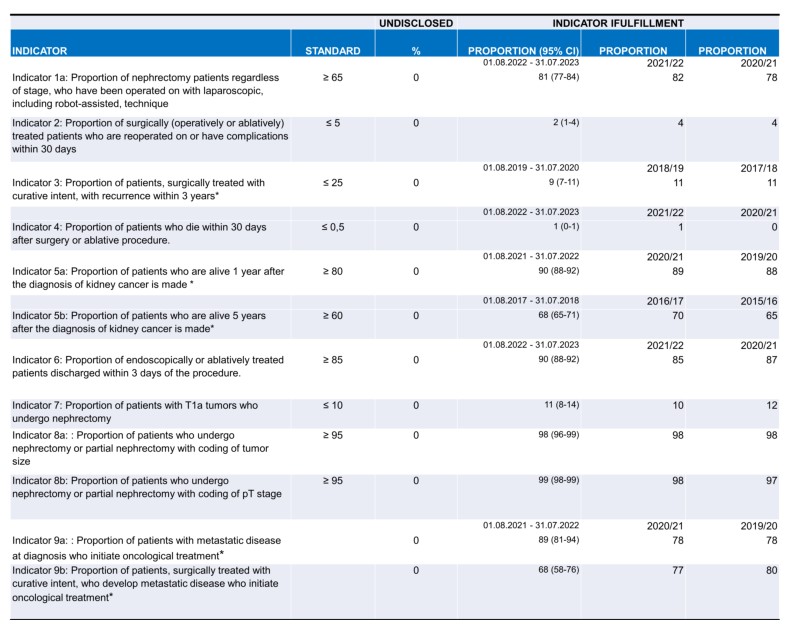
Table 2. An overview of the indicator results. * Time-lagged indicator, current year is moved back in time to allow for necessary follow-up time.
The treatment of kidney cancer is primarily surgical, and surgical treatment continues to move towards an increased proportion of kidney-preserving procedures. The standards mentioned in table 1 and 2 are based in the current literature. During the current period, 931 patients were treated surgically (operation or ablation), and of these, 55% underwent kidney-preserving interventions. The vast majority of kidney cancer patients are now operated on using laparoscopic surgery. The standard was > 65%, but with some variation between the five Danish regions (68-96%). The proportion of patients operated on using laparoscopic techniques has increased from 65 to 85 percent in the last five years alone. Nine out of ten patients operated on laparoscopically can be discharged within three days, and very few patients experience complications or require reoperation.
During the years, the chairperson in Darenca has an audit with the chairs of the individual departments if the report shows that there was an opportunity for improvement. This has shown to be essential because it clearly demonstrated that performing minimal invasive surgery was for the benefit of the patients. Some departments performed most cases by open surgery and performed nephrectomy for T1A tumors. The chairs could see the advantage of this development because it gave the opportunity to get teams that were more robust, better equipment and facilities at the theater. From 2024 and onwards the indicatorn 1 has been changed so that the standard is now that > 85% of the surgeries should be performed robotic assisted or laparoscopically. This demonstrated how collaboration in the register has been a lever for the development of professional work - both locally and nationally.
There length of hospital stay after the procedures was short (< 3 days), while the postoperative morbidity of 3% and the postoperative mortality of just over 0.5% within 30 days were stably low (Table 2). Morbidity and mortality were at an international level. 10 Among patients with small tumors (stage T1a), 11% underwent nephrectomy rather than kidney-conserving treatment demonstrating a decreasing proportion of nephrectomies over time (Figure 3). The result has become very close to the defined standard for this indicator and was uniform between the regions. This evolution was created in the same fashion as mentioned above.
Nephrectomies were performed laparoscopically incl. robot-assisted 81% (last year 82%) with some variation between departments. The proportion of endoscopically or ablatively treated patients who were discharged within 3 days after the procedure is 90%, also with some variation between departments. A positive development has been seen nationally and in 4 of the 8 treating departments. Part of the report's data is drawn directly from the LRP, to which data from the country's pathology departments is automatically transferred. As in previous annual reports, there was a high overall data completeness for coding pT stage and tumor size. In 2023, 97% of all nephrectomies and partial nephrectomies/renal resections were registered with a pT stage with very low variation among pathology departments. Tumor size (tumor diameter) was reported for 98% of all nephrectomies/partial nephrectomies/renal resections, also with a small variation. In general, a great effort is being made to classify the histological subtype, which has prognostic and therapeutic significance, as only 5% were recorded as 'unclassifiable renal cell carcinoma', which is in line with international calculations10 .
This annual report has compiled indicators for the second time describing the oncological treatment of kidney cancer, which show that 89% of newly diagnosed patients with metastatic disease who were seen at an oncological treatment facility receive oncological treatment. Work on improving the data quality on oncological treatment decisions is still being optimized. The DaRenCa group has decided to have two new indicators starting from year 2024 report: MDT and the use of pembrolizumab.
CONCLUSION
Through systematic work with quality results, interdisciplinary collaboration and the introduction of new treatments, we have observed a uniform treatment and increased survival in patients with kidney cancer.Availability of data and Materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.Conflicts of interest
All authors declare no conflicts of interest, financial or otherwise, related to this manuscript.Funding Sources
This study was not supported by any sponsor or funder.Contributions
All authors played a substantial role in conception, design, acquisition, analysis, interpretation, writing, and critical review of the manuscript. All authors approved the final content and accepted
REFERENCE
1) Nørgaard M, Johnsen SP. How can the research potential of the clinical quality databases be maximized? The Danish experience. J Intern Med. 2016 Feb;279(2):132-40
2) Sørensen HT, Pedersen L, Jørgensen J, Ehrenstein V. Danish clinical quality databases - an important and untapped resource for clinical research. .Clin Epidemiol. 2016 Oct 25;8:425-427
3) https://www.sundk.dk/
4) Simoni AH, Ahlstrøm LMV, Ording AG, Iversen LH, Johnsen SP, Jensen JW et all.. Quality indicators and development targets in the national clinical quality registries in cancer care and screening. BMJ Open Qual. 2025 Jan 4;14(1):e003019.
5) Sundhedsdatastyrelsen. Kliniske kvalitetsdatabaser [internet]. København: Sundhedsdatastyrelsen; 2021. Available from: https://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-kliniske-kvalitetsdatabaser
6) Green A. Danish clinical databases: an overview. Scand J Public Health. 2011;39(7 Suppl):68–71.
7) Institute of Medicine (IOM). Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C: National Academy Press; 2001.
8) Sundhedsvæsenets kvalitets- og patientsikkerhedsbegreber & Metodehåndbog i kvalitetsudvikling og patientsikkerhed. Dansk Selskab for Kvalitet i Sundhedssektoren. 3. udg., september 2018. https://dsks.dk/wpcontent/uploads/2018/09/Metodeh%C3%A5ndbog-12-09-2018.pdf
9) Danckert B, Horsbøl TA, Andersen O, Dalton SO, Christensen J, Rasted M, et al.. Registrations of Patients with Renal Cell Carcinoma in the Nationwide Danish Renal Cancer Database versus the Danish Cancer Registry: Data Quality, Completeness and Survival (DaRenCa Study-3). .Clin Epidemiol. 2020 Jul 27;12:807-814
10) Ljungberg B, Albiges L, Abu-Ghanem Y, et al.. European Association of Urology guidelines on renal cell carcinoma: The 2022 update. Eur Urol. 2022;82(4): 399–410. https://doi.org/10.1016/j.eururo.2022.03.006



