ASCO GU 2023 Sessions:
![]() DOWNLOAD PDF
DOWNLOAD PDF
RECOMMENDED ABSTRACTS in RENAL CANCERS

 Some Other Text
Some Other Text

These recommended abstracts from ASCO23 Annual meeting have been selected by Robert A. Figlin, MD, Editor-in- Chief of the Kidney Cancer Journal. The chosen abstracts provided here highlight some of the most important trends in ongoing trials and reflect the foremost research and strategies from latest clinical trials that impact the current standard of care in renal cancer.





ABSTRACT LBA 602
- Results from phase 3 study of 89Zr-DFO-girentuximab for PET/CT imaging of clear cell renal cell carcinoma (ZIRCON). Allison May et al.Background: The increasing detection of renal masses presents a significant patient management challenge. Diagnostic options include cross-sectional imaging, which cannot reliably differentiate benign and malignant renal masses, and biopsy, which is invasive and subject to sampling errors. These limitations highlight the unmet need for accurate noninvasive techniques to guide patient management. Girentuximab is a monoclonal antibody that targets carbonic anhydrase IX (CAIX), an enzyme highly expressed in clear cell renal carcinoma (ccRCC). Radiolabeled 89Zr-DFO-girentuximab (TLX250-CDx) is highly specific for CAIX and can aid differentiation between ccRCCs and other renal lesions. The ZIRCON study evaluated the performance of TLX250-CDx PET/CT for detection of ccRCC in adult patients with indeterminate renal masses (IDRM).
Methods: ZIRCON was an open-label, multicenter clinical trial. Patients with an IDRM (≤ 7 cm; tumor stage cT1) who were scheduled for partial nephrectomy within 90 days from planned TLX250-CDx administration were eligible. Enrolled patients received a single dose of TLX250-CDx IV (37 MBq ± 10%; 10 mg girentuximab) on Day 0 and underwent PET/CT imaging on Day 5 (± 2 days) prior to surgery. Blinded central histology review determined ccRCC status. The coprimary objectives were to evaluate both the sensitivity and specificity of TLX250-CDx PET/CT imaging in detecting ccRCC in patients with IDRM, using histology as the standard of truth. Key secondary objectives included sensitivity and specificity of TLX250-CDx PET/CT imaging in the subgroup of patients with IDRM ≤ 4 cm (cT1a). Other secondary objectives included positive and negative predictive values, safety, and tolerability. The Wilson 95% confidence intervals (CI) lower bound for sensitivity and specificity had to be > 70% and 68% respectively for ≥ 2 independent readers to declare the study successful.
Results: 300 patients received TLX250-CDx; mean age was 62 ± 12 y; 71% were males. Of 288 patients with central histopathology of surgical samples, 193 (67%) had ccRCC, and 179 (62%) had CT1a; Of 284 evaluable patients included in primary analysis, the average across all 3 readers for sensitivity and specificity was 86% [80%, 90%] and 87% [79%, 92%] respectively for coprimary endpoints; and 85% [77%, 91%] and 90% [79%, 95%] respectively for key secondary endpoints. For all readers, the lower boundaries of 95% CI for coprimary and key secondary endpoints were > 75%. For all evaluable patients, positive and negative predictive values were ≥ 91.7% and ≥ 73.7%, respectively. Of 263 treatment-emergent adverse events (TEAEs), 2 TEAEs were treatment related.
Conclusions: This study confirms that TLX250-CDx PET/CT is well tolerated and can accurately and noninvasively identify ccRCC, with promising utility for designing best management approaches for patients with IDRM. Clinical trial information: NCT03849118.
ABSTRACT 604
Treatment-free survival (TFS) outcomes from the phase II study of nivolumab and salvage nivolumab + ipilimumab in advanced clear cell renal cell carcinoma (aRCC) (HCRN GU16-260-Cohort A). Atkins MB et alBackground: Treatment with immunotherapy can be associated with prolonged disease control after discontinuation without the need for further anticancer therapy. Toxicity from therapy can also persist after cessation. TFS with and without toxicity can characterize survival time. Significant TFS was reported for CheckMate 067 trial in pts with metastatic melanoma (Regan et al JITC 2021) and CheckMate 214 trial for pts with aRCC (Regan et al CCR 2021), but treatment was often halted for toxicity rather than a pre-defined treatment endpoint. We therefore sought to assess TFS in the HCRN GU16 260 trial, which was designed to reduce toxicity and to cap immunotherapy duration (Atkins et al JCO 2022).
Methods: Data were analyzed from 128 patients (pts) with clear-cell aRCC treated with first-line nivolumab (NIVO) monotherapy for up to 2 years. As part of the protocol, salvage nivolumab/ipilimumab (NIVO/IPI) for up to 1 year was provided to eligible patients with disease progression at any point or stable disease at 48 weeks (28% of pts). TFS was defined as the area between Kaplan-Meier curves for time from registration to protocol therapy cessation and for time from registration to subsequent therapy initiation or death, estimated from 36-month (mo) mean times. The time on treatment or off treatment with grade 3+ treatment-related adverse events (TRAEs) was also captured. Results: At 36 mos from enrollment, 68.3% of pts were alive: 96.8% of IMDC favorable-risk (FAV) pts and 56.6% of those with intermediate/poor-risk (I/P), respectively. The 36-mo mean time on protocol therapy was 11.5 mos (16.0 mos for FAV pts and 9.6 mos for I/P pts). The 36-mo mean TFS for the whole population was 9.4 mos. For FAV pts the mean TFS was 12.9 mos, of which TFS with grade 3+ TRAEs was 1.5 mos. For I/P pts, the mean TFS was 8.0 mos, of which TFS with grade 3+ TRAEs was 1.0 mos. At 36 mos, 65.6% of FAV pts and 27.1% of I/P pts were alive and second-line treatment-free.
Conclusions: NIVO monotherapy with salvage NIVO/IPI in non-responders is an active treatment approach in treatment-naïve pts with aRCC and results in substantial TFS and toxicity-free TFS. TFS was particularly noted in pts with FAV disease, further supporting the use of an immunotherapy-only regimen in this population. Clinical trial information: NCT03117309.
ABSTRACT 608
- Biomarker analysis from the phase 3 CheckMate 9ER trial of nivolumab + cabozantinib v sunitinib for advanced renal cell carcinoma (aRCC). Choueiri TK et al.
Background: Prior studies investigated determinants of anti–PD-L1 ± anti-angiogenic response in aRCC. The mechanisms underlying response to first-line anti–PD-1 + VEGF-targeted therapy (anti-VEGF) remain largely unknown. In this exploratory post hoc analysis we conducted an analysis of anti–PD-1 (N) + anti-VEGF (C) v S by assessing potential predictive biomarkers for efficacy in CheckMate 9ER. Analyzed biomarkers included CD8 T-cell frequency (% of total tumor cells, CD8%), CD8 topology, PD-L1 immunohistochemistry, gene set enrichment analysis (GSEA), and 7 gene expression signatures (GES).
Methods: Progression-free survival (PFS) and overall survival (OS) were evaluated by tumor PD-L1 expression (< 1% or ≥ 1%), CD8% (low, medium, high by tertiles), and CD8 topology phenotype (cold, excluded, inflamed), and assessed for association using Kaplan–Meier (KM) methods with log-rank test (PD-L1 and CD8), and Cox proportional hazard (Cox PH) models (CD8) (to avoid arbitrary or biased categorization of continuous-valued predictor variables, a potential limitation of KM analyses). Pre-ranked GSEA was performed to assess enrichment for hallmark gene sets using all genes ranked by interaction effect estimates derived using Cox PH regression. Seven GES (Angio, Myeloid, Teff, TIS, Interferon-γ, EMT8, Javelin), including several previously found to be predictive of anti-PD-L1 ± anti-VEGF outcomes, were assessed for association with PFS within treatment arms.
Results: At 44 mo median follow-up, median PFS and OS were improved with N+C v S regardless of PD-L1 status. PD-L1 < 1% v ≥ 1% was associated with longer median PFS in the S arm only (P = 0.00045). In KM analyses, higher CD8% was associated with improvements in PFS with N+C, but not S. Of the 410 patients (pts) in the CD8 topology analysis, the predominant CD8 phenotype was inflamed (46.8%), then cold (40.5%) and excluded (12.7%). CD8 topology supported an association between the inflamed phenotype and improved survival outcomes with N+C v S (PFS, P < 0.0001; OS, P = 0.00097). However, these associations were not confirmed in Cox PH models. Common hallmark gene sets with positive (p) or negative (n) enrichment (with false discovery rate < 0.05) in genes associated with longer PFS and OS with N+C v S included oxidative phosphorylation, hypoxia, adipogenesis, P53 pathway (p), and E2F targets (n). Pts receiving N+C had longer median PFS with high Angio GES v medium and low Angio GES (P = 0.019). However, all 7 GES tested, including Angio GES, were not predictive for N+C outcomes in Cox PH models.
Conclusions: In this exploratory post hoc analysis, biomarkers previously found to be predictive of anti-PD-L1 ± anti-VEGF outcomes, including established GES, were not predictive of efficacy with anti-PD-1 + anti-VEGF (N+C) using Cox PH models. This suggests that key determinants of response to anti–PD-1 v anti–PD-L1 therapies may differ. Clinical trial information: NCT03141177.
Abstract CTR11
- - First-line nivolumab plus ipilimumab (NIVO+IPI) versus sunitinib (SUN) in patients with long-term survival of ≥5 years in the CheckMate 214 trialTannir NM, Motzer RJ, McDermott DF, Plimack ER, George S, Amin A, Tykodi SS, Srinivas S, Carthon B, Hutson TE, Lee CW, Desilva H, Jiang R, Hammers HJ
Background: First-line NIVO+IPI provided long-term survival benefits versus SUN in patients with advanced renal cell carcinoma (aRCC) after 5 years follow-up in CheckMate 214. Methods: Patients with clear cell aRCC were randomized to NIVO 3 mg/kg plus IPI 1 mg/kg Q3W×4 then NIVO 3 mg/kg Q2W versus SUN 50 mg QD (4 weeks of 6-week cycles). In this post hoc exploratory analysis, outcomes in patients with overall survival ≥5 years (long-term survivors; LTS) were assessed by IMDC risk (intermediate/poor [I/P-risk] and favorable [FAV-risk]).
Results: Overall, 163/425 I/P-risk and 73/125 FAV-risk patients in the NIVO+IPI arm versus 112/422 I/P-risk and 59/124 FAVrisk patients in the SUN arm were LTS. Baseline characteristics generally did not distinguish LTS from intent-to-treat patients with NIVO+IPI, except target lesions were smaller and fewer patients had bone metastases. Regardless of risk group in LTS, there were more durable and complete responses with NIVO+IPI versus SUN. Fewer LTS required subsequent systemic therapy with NIVO+IPI versus SUN, and most patients in the SUN arm with subsequent therapy received NIVO monotherapy regardless of risk. More LTS who responded experienced a treatment-free interval with NIVO+IPI versus SUN. Treatment-related adverse events leading to discontinuation did not preclude surviving ≥5 years.
Conclusions: These results highlight the long-term clinical benefits and continued durability of response observed with NIVO+IPI in patients across a spectrum of baseline characteristics and regardless of IMDC risk.
ABSTRACT 614
- - Impact of race and payor status on patterns of utilization of partial and radical nephrectomy in patients with localized renal cell carcinoma (RCC)Barragan-Carrillo R et al.
Background: Racial minorities experience intersecting forms of marginalization and suffer significant healthcare disparities. Prospective trials have shown similar outcomes with partial and radical nephrectomy among patients with localized RCC (Van Poppel et al Eur Urol 2011), and multiple studies suggest increasing use of the former technique (Breau et al Can J Urol 2020). We hypothesize that patients from minority groups, as well as those with non-private insurance, will have less access to this specialized procedure and therefore have a higher rate of radical nephrectomy. Methods: We utilized the California Office of Statewide Health Planning and Development (OSHPD) database that collects information from all inpatient admissions, emergency room visits and inpatient/outpatient procedures in the state. All patients undergoing nephrectomy (both partial and radical) were identified from Jan 1, 2012 to Dec 31, 2018 using CPT and ICD-9/10 codes to identify patients. Demographic data was collected with specific attention to race and payor status. Univariate and multivariate analyses were conducted to determine the association between demographic data and procedure type. Results: In total, 31,093 patients were identified; 57% were males, with a mean age of 58 years. Among these, 16,142 (51.9%), 8,645 (27.8%), 2,795 (9.0%), 2,032 (6.5%) and 1,479 (4.8%) were characterized as White, Hispanic, Asian, Black and other, respectively. Partial nephrectomy and radical nephrectomy were performed in 15,840 (50.9%) and 15,253 (49.1%) of patients. By race, partial nephrectomy was performed in 8,576 (53.1%), 4,107 (47.5%), 1,286 (46.0%), 1,124 (55.3%) and 747 (50.5%) of White, Hispanic, Asian, Black and other patients, respectively (p<0.001). Use of partial nephrectomy also differed among patients based on payor status, with rates of 6,800 (56.4%), 5,036 (43.9%), 1,817 (38.3%) and 2,187 (77.7%) among patients with private, Medicare, indigent coverage (e.g., MediCal or Medicaid) and other insurance, respectively (p<0.001). On multivariate analysis controlling for age, gender, comorbidities and frailty, race was independently associated with type of nephrectomy procedure. Conclusions: Our study confirms that race and payor status may have an influence on utilization of partial versus radical nephrectomy, with the highest rate of partial nephrectomies among Whites and patients with private insurance. Although there are multiple potential confounders (e.g., latency of diagnosis and resulting tumor size/complexity), it is possible that access to care may be an important driver of these disparities.
ABSTRACT 629
- - Estimated cost of adverse event (AE) management for patients (pts) with metastatic renal cell carcinoma (mRCC) treated with a VEGFR TKI.Jeffrey Thomas Yorio et al
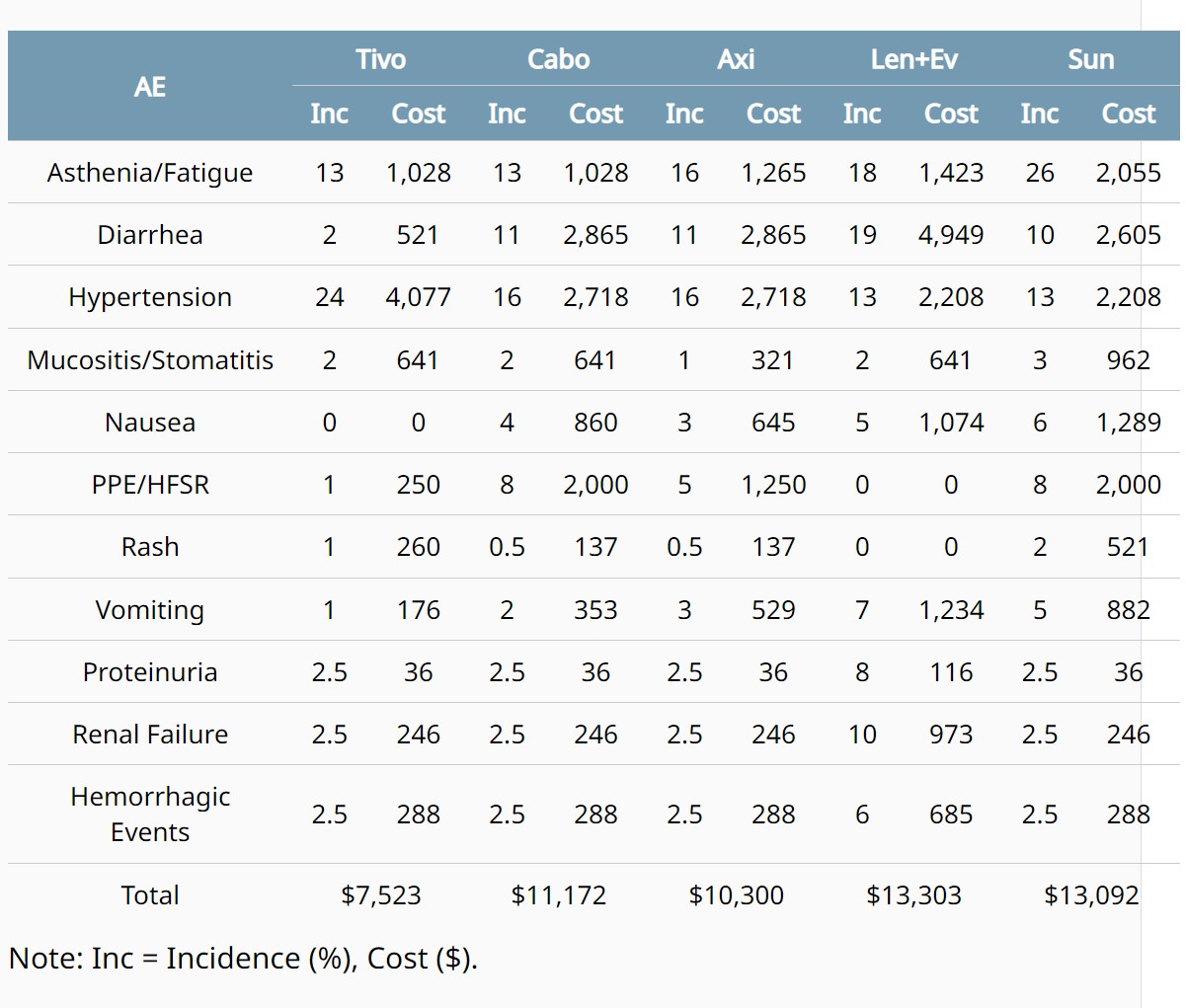
Background: Nearly all mRCC pts will be treated with one or more VEGFR TKI, alone or in combination with an immunotherapy (IO) or mTOR-inhibitor, during their therapeutic journey and may experience associated AEs. Evidence from the tivozanib (tivo) TIVO-3 trial suggests that median duration of clinically relevant VEGFR TKI class effect AEs is ≤90 days (range 14-90). Cost of AE management has not been clearly quantified to date. This study aimed to estimate how differences in TKI AE profile may impact treatment cost efficiency, particularly in later line (3L+) settings. Methods: iKnowMed EMR was used to identify mRCC pts from USON or Onmark Network, with matched 3rd party insurance claims, that initiated VEGFR TKI treatment between Jan 2015 and Mar 2021. First occurrence of each VEGFR TKI class effect AE was indexed, and associated costs for 90-day (longest median AE duration) follow-up was captured. To assess burden across different TKIs, average per-patient AE management cost was calculated using incidence data from trials supporting FDA approvals, and weight-adjusted to estimated number of commercially insured 3L/4L pts in a 1,000,000-member plan. Results: 5,958 mRCC pts were identified, of which 4,464 were on at least one TKI regimen. Among those, 1,777 experienced an index AE; 1,072 successfully matched to claims data [median 69 years (range 25-94); 55% ECOG PS 0/1; 69% male], accounting for 1,667 unique index AE cases. Most were on cabozantinib (cabo), axitinib (axi), or pazopanib; lenvatinib (len), sunitinib (sun), and sorafenib were also represented. >80% were TKI only, with the rest TKI+IO (18%) or TKI+mTOR (6%). AE costs largely originated from outpatient visits (range 38-77%), excluding renal failure (58% inpatient). Mean cost per AE ranged from $76 (proteinuria) to $1,687 (mucositis/stomatitis). Overall, estimated costs of managing VEGFR TKI class effect AEs in 3L/4L showed lowest resource burden with tivo, and highest with len+everolimus (len+ev; Table). Conclusions: Average VEGFR TKI AE management costs derived from real-world mRCC pts demonstrated differences in healthcare resource burden, with overall anticipated cost dependent on TKI regimen utilized.
Sum of managing AEs suggests potential cost offsets with use of tivo in 3L+ for mRCC.
ABSTRACT 634
- Financial toxicity from first-line TKI plus IO therapies for advanced renal cell carcinoma.David Joseph Benjamin et al
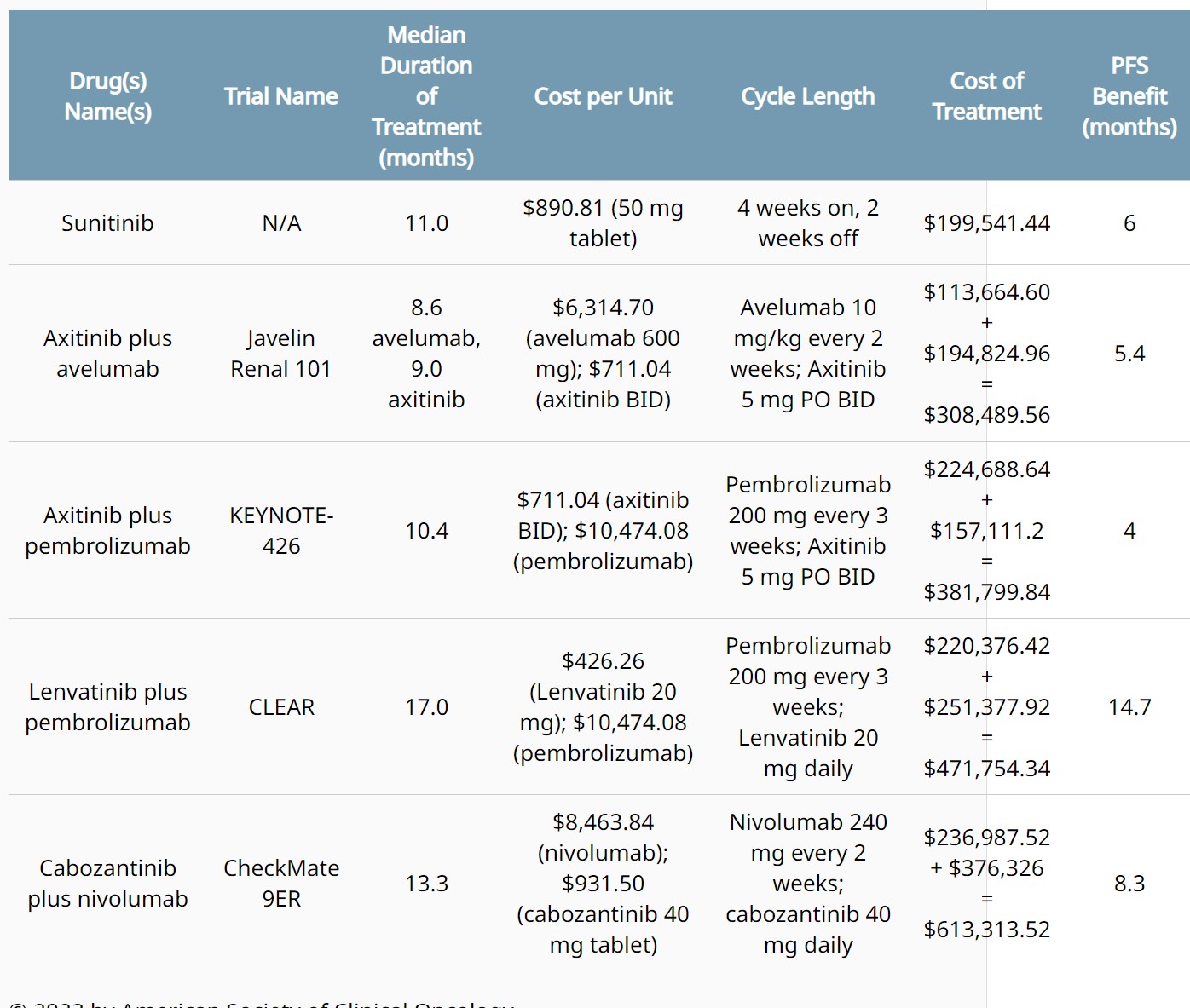
Background: Since the approval of sunitinib for the treatment of advanced renal cell carcinoma (RCC), the first-line treatment landscape has drastically altered with combination tyrosine kinase inhibitor (TKI) plus immunotherapy (IO) regimens. Despite improvements in survival, it remains unclear if these therapies are cost prohibitive for patients or hospitals compared to prior standard of care sunitinib. Methods: Approved TKI plus IO therapies were identified using the US FDA Oncology Announcements website and confirmed with NCCN Guidelines (Version 3.2023). Cost per unit was identified using public data (Lexicomp or manufacturer’s website). We calculated total cost of each treatment regimen using the cost per unit for each therapy and the median duration on treatment as reported in each combination therapy's clinical trial publication. Results: Average PFS benefit from combination TKI plus IO therapies was 8.1 months (range 4-14.7) in comparison with sunitinib. Average cost of TKI plus IO therapy was $443,839.32 compared with $199,541.44 for sunitinib therapy. Conclusions: Average increase in cost of TKI plus IO therapy compared to sunitinib was $244,297.88. For every month of PFS benefit with TKI plus IO combination therapy, there was on average $30,160.23 cost added per month. These increased costs may be prohibitive for many patients and hospitals, particularly in low- and middle-income countries (LMICs).
List of FDA approvals for treatment (TKI plus IO therapies and sunitinib) of advanced RCC.


ABSTRACT 647
- - Cabozantinib in the elderly with metastatic renal cell carcinoma undergoing geriatric G8 screening test: A prospective multicenter observational study (ZEBRA/MEET-URO 9).Basso U et al.
Background: Cabozantinib (CABO) is an oral tyrosine kinase inhibitor registered for the treatment of metastatic renal cell carcinoma (mRCC) for the first or subsequent lines. Tolerability in real world elderly patients is poorly documented. G8 is a short test for vulnerability gaining increased interest as a screening tool for trials in geriatric oncology. Methods: ZEBRA/MEET-URO 9 was a prospective multicenter study of safety and activity of CABO administered to pts ≥70 years with mRCC, either in the first or subsequent lines of treatment, until progression or unacceptable toxicity. All pts underwent G8 score at baseline, with a cut-off for vulnerability of 14 or below. Data on tolerability and activity were collected prospectively after signature of informed consent. Results: A total of 104 pts started CABO at 13 Italian Centers, 38,5% as first line. Median age was 75.8 yrs (range 70.2-87.4 yrs, 26 pts ≥80 yrs), 73.1% males. IMDC score was good 19.2%, intermediate 53.9%, poor 26.9%. Primary tumor had been removed in 82.7% of pts, histology was clear cell 78.8%, papillary 8.7%, chromophobe 5.8%, unclassified 6.7%. G8 score was ≤14 in 65.4% of pts. Up-front dose reduction of CABO was more frequent in pts with low G8 score (79.4 vs 41.7%, p=0.003), but eventually the majority of pts (91.4%) underwent dose reductions of CABO. After a median treatment of 6.4 months (0.5-26.1 months), 38.4% of pts developed G3-4 toxicities, 22.1% interrupted treatment due to adverse events, 2.8% (3 pts) died due to cardiovascular or thromboembolic events. Median PFS was 7.6 months (95% CI=5.8-12.6 months) in first line, 10.0 months (5.8-15.6) in second or further lines, median OS was 20.1 months (11.1-not reached) and 15.6 months (12.5-not reached), respectively. G8 score ≤14 did not correlate with rate of temporary interruptions >7 days, hospitalization, incidence of G3-5 toxicities, as well as with PFS. Pts with G8 score ≤14 had a trend for reduced OS, but difference was not statistically significant both in the first and further lines of treatment. Conclusions: Screening G8 test was positive in more than a half of pts, underlying the need for detailed geriatric assessment and increased clinical monitoring of such patients. A G8 score ≤14 correlated with up-front dose reduction of CABO but not with G3-5 toxicities probably due to the high rates of dose reductions in the whole cohort. Correlation between low G8 score and OS could not be demonstrated in this population.
ABSTRACT 659
- - Correlating HLA variants and the emergence of immune-related adverse events (irAEs) from ipilimumab/nivolumab (I/N) in patients (pts) treated on CheckMate 214.Voss MH et al.
Background: Combination therapy with I/N can achieve long-term benefit in a sizable proportion of RCC pts, but broad application has been challenged by a high incidence of irAEs. Host features affecting antigen presentation are candidate determinants of irAE pathogenesis, and isolated reports have linked Human Leukocyte Antigen (HLA) variations to organ- specific irAEs. We analyzed data for pts treated on a phase 3 trial to investigate association of class 1 HLA variants with development of irAEs from I/N and develop a tool to predict risk for toxicity. Methods: We analyzed germline HLA + clinical data for 472 pts receiving I/N vs sunitinib (SUT). Based on allelic variants for HLA-A and B pts were categorized into 12 established HLA "Super-Types" (ST), sets of HLA variants with largely overlapping peptide binding specificity. We conducted uni- and multivariate (UV; MV) Cox proportional hazards regression to correlate HLA-ST variables with time to treatment-related AEs (TTrAE, grade 2+) in I/N pts using the Kaplan Meier method. Several MV HLA-ST models were developed on a discovery set (DISC; 2/3 random sample of I/N pts without replacement ) and compared using model concordance (c-index) on a validation set (VAL; remaining 1/3). Model coefficients were used to create a “HLA-ST score” that could be computed for individual pts. Results: 235 pts and 237 pts received I/N vs SUT and had available HLA data, respectively. On UV analysis for I/N, HLA-B07 ST had protective association TTrAE (HR= 0.65, 95% CI: 0.46,0.90; p= 0.010), while B62 associated adversely (HR=1.64, 95% CI: 1.12, 2.40; p=0.014). Relevant to the development of our model we identified interactions between several pairs of HLA ST. The HLA ST model with best performance in DISC (c-index= 0.606) and VAL (c-index=0.595) integrates B07, B62, A01, B08, and two interactions: B07-B08 and B07-A01. The model-generated HLA-ST score was significantly associated with TTrAE after adjusting for race, BMI, region, PDL1 status, and MSKCC risk score (DISC p<0.001; VAL p=0.028). No association with TTrAE was seen in SUT treated pts (p=0.655), neither in UV nor MV model. I/N-treated patients with HLA-ST score dichotomized >= vs < median had significantly different TTrAE both in DISC (p=0.004) and VAL (p=0.009); again, no difference was observed in the SUT arm (p=0.5). The weighted HLA-ST score had no association with PFS or OS for I/N pts. Conclusions: In this large sample of I/N-treated patients, class I HLA variants were associated with the risk of developing irAEs. We developed a HLA ST based score that correlated with treatment toxicity independent of relevant clinical and demographic features. No association with TTrAE was seen in SUT-treated pts. These results highlight the potential of characterizing germline features to predict immune related toxicity upfront and deserves further study. Clinical trial information: NCT02231749.
ABSTRACT 674
- - Survival outcomes of metastasis-directed therapy for solitary sites of metastatic clear cell renal cell carcinoma.Akeem Ronell Lewis, et al.
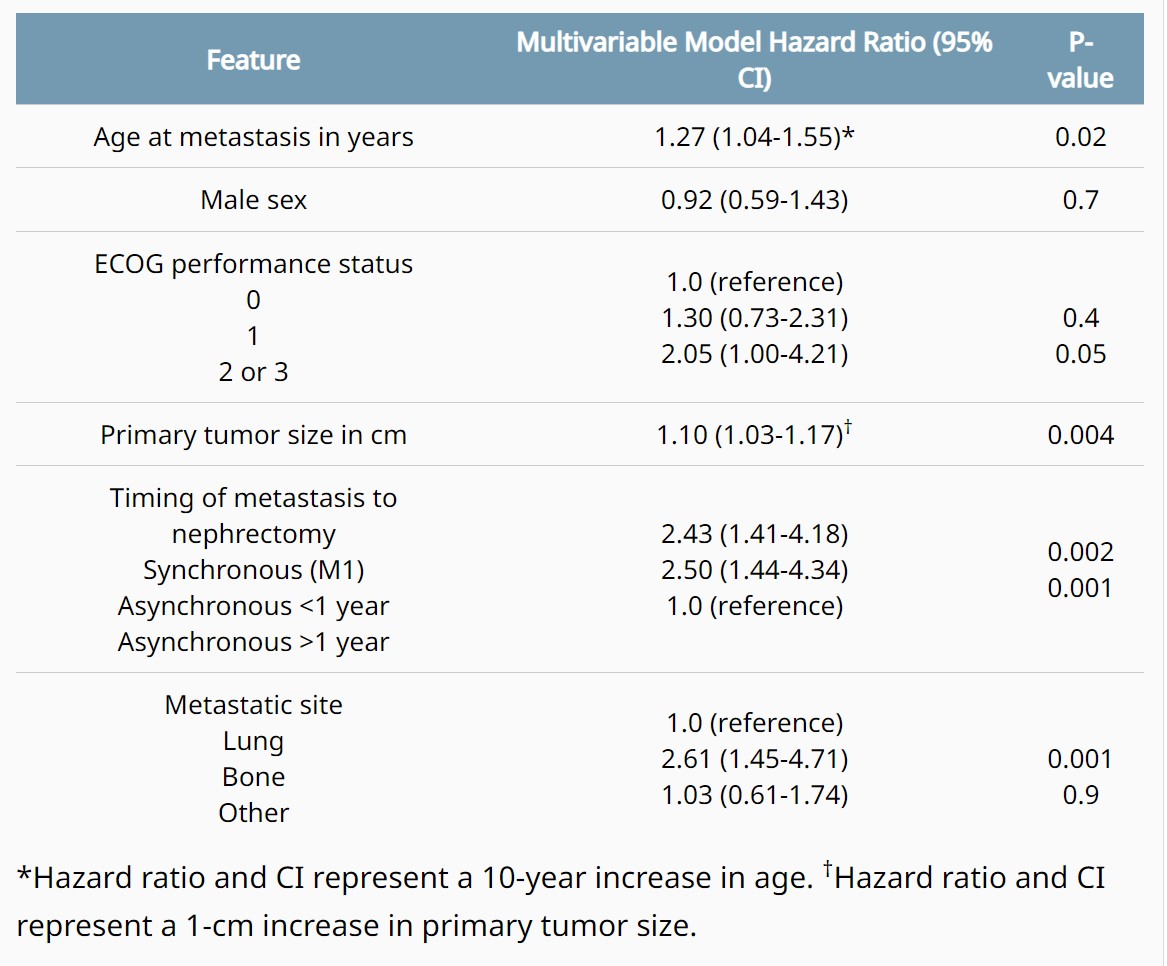
Background: Metastasis directed therapy (MDT) is associated with improved cancer-specific survival and delay in use of systemic therapy for metastatic clear cell RCC (mccRCC). Although the benefits of MDT may differ based on organ site of metastasis due to differences in disease biology, survival based on site of metastasis remain underexplored. We aim to evaluate survival outcomes of patients who underwent MDT for solitary sites of mccRCC. Methods: The Mayo Clinic Nephrectomy Registry was queried to identify adults undergoing radical or partial nephrectomy for unilateral, sporadic ccRCC from 2000 to 2019 with a single site of metastasis treated with MDT including complete metastasectomy or radiation, in lieu of systemic therapy. Overall and cancer-specific survival were estimated using the Kaplan-Meier method, with the duration of follow-up calculated from the date of metastasis to the date of death or last follow-up. Associations with time to death from RCC were evaluated using Cox proportional hazards regression models and summarized with hazard ratios and 95% confidence intervals (CIs). Results: In this cohort of 207 mccRCC patients, 152 underwent complete metastasectomy and 55 underwent radiation. 133 died at a median of 2.7 years (IQR 1.2-4.7) following metastasis, including 105 who died from RCC at a median of 2.2 years (IQR 1.0-3.9). The median duration of follow-up for the 74 patients who were still alive at last follow-up was 8.1 years (IQR 3.7-12.1). Overall survival rates (95% CI) at 2, 4, 6, 8, and 10 years following metastasis were 73% (69-79), 54% (48-62), 45% (38-52), 37% (30-44), and 32% (26-40), respectively; cancer-specific survival rates were 75% (69-81), 56% (50-64), 47% (41-55), 44% (37-52), and 42% (35-50), respectively. Age, poor performance status, presence of synchronous metastasis and asynchronous metastasis <1 year from nephrectomy, tumor size, and bone metastasis were associated with death from RCC (table). Conclusions: These findings provide useful survival benchmarks to patients who are considering MDT as a therapeutic option. In addition, synchronous and asynchronous metastasis <1 year from nephrectomy, poor performance status, and bone metastasis are significantly associated with worse survival from mccRCC.


ABSTRACT 684
- - Cabozantinib in combination with atezolizumab in non-clear cell renal cell carcinoma: Extended follow-up results of cohort 10 of the COSMIC-021 study.Bradley Alexander McGregor et al.
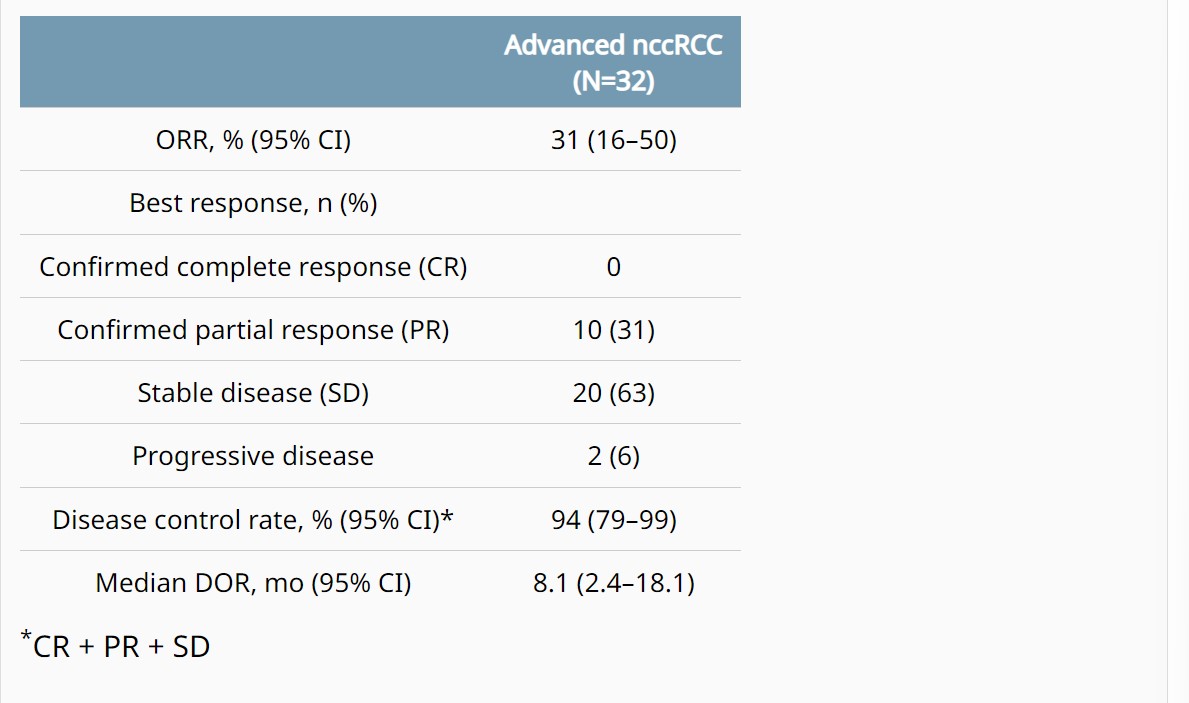
Background: In the COSMIC-021 phase 1b study (NCT03170960) evaluating cabozantinib plus atezolizumab in advanced solid tumors, this combination therapy demonstrated encouraging clinical activity in patients with advanced non-clear cell renal cell carcinoma (nccRCC) with a median follow-up of 13 mo (Pal. JCO 2021). Results after extended follow-up in nccRCC are presented. Methods: Patients with advanced nccRCC and ECOG PS 0/1 who had ≤1 prior VEGFR-targeting tyrosine kinase inhibitor (TKI) were eligible. Prior treatment with TKIs targeting MET or immune checkpoint inhibitors was not allowed. Patients received cabozantinib 40 mg PO QD plus atezolizumab 1200 mg IV Q3W until unacceptable toxicity or progression; dose reductions of cabozantinib (40 mg QD to 20 mg QD, then to 20 mg QOD) were permitted to manage adverse events. The primary endpoint was objective response rate (ORR) per RECIST v1.1 by the investigator; other endpoints included safety, duration of response (DOR), PFS, and OS. Results: The study enrolled 32 patients with nccRCC (2 from dose escalation phase, and 30 from expansion phase of the study): median age, 62 y; male, 81%; ECOG PS 0/1, 75%/25%; histology, papillary/chromophobe/clear cell/other, 47%/28%/3%/22%; sarcomatoid feature, 13%; IMDC risk favorable/intermediate/poor, 50%/41%/9%; ≥3 tumor sites, 56%; tumor sites, lung/kidney/bone/liver, 50%/25%/16%/16%; prior nephrectomy, 63%; prior VEGFR TKI, 22%; 0/1 lines of prior therapy (locally advanced/metastatic setting), 81%/19%. As of July 21, 2022, median follow-up was 37.2 mo (range 32.1–58.5) with 5 (16%) patients remaining on study treatment. ORR by investigator was 31% (all PRs) and disease control rate was 94% (Table); median DOR was 8.1 mo. Median PFS was 9.3 mo (95% CI 5.5–12.3), and median OS was not reached (95% CI 23.0–NE). PFS and OS estimates at 12 mo were 34% and 84%, respectively; 24-mo estimates were 6% and 70%. Treatment-related AEs occurred in 97% (grade 3/4, 53%); the most common AEs included diarrhea (69%), palmar-plantar erythrodysesthesia (50%), fatigue (44%), dysgeusia (41%), hypertension (31%) and nausea (31%). One grade 5 treatment-related AE of pulmonary hemorrhage occurred. Treatment-related AEs leading to discontinuation of both study treatments occurred in 13% of patients. Conclusions: Extended 3-year follow-up reinforces the encouraging clinical activity of cabozantinib plus atezolizumab in advanced nccRCC with a manageable safety profile. Clinical trial information: NCT03170960.


ABSTRACT 714
- - Evaluation of PBRM1, PD-L1, CD31, and CD4/CD8 ratio as a predictive signature of response to VEGFR-TKI–based therapy in patients with metastatic renal cell carcinoma (mRCC) with IMDC intermediate prognosis: Results from the APAChE-I Study.Chiara Ciccarese et al.
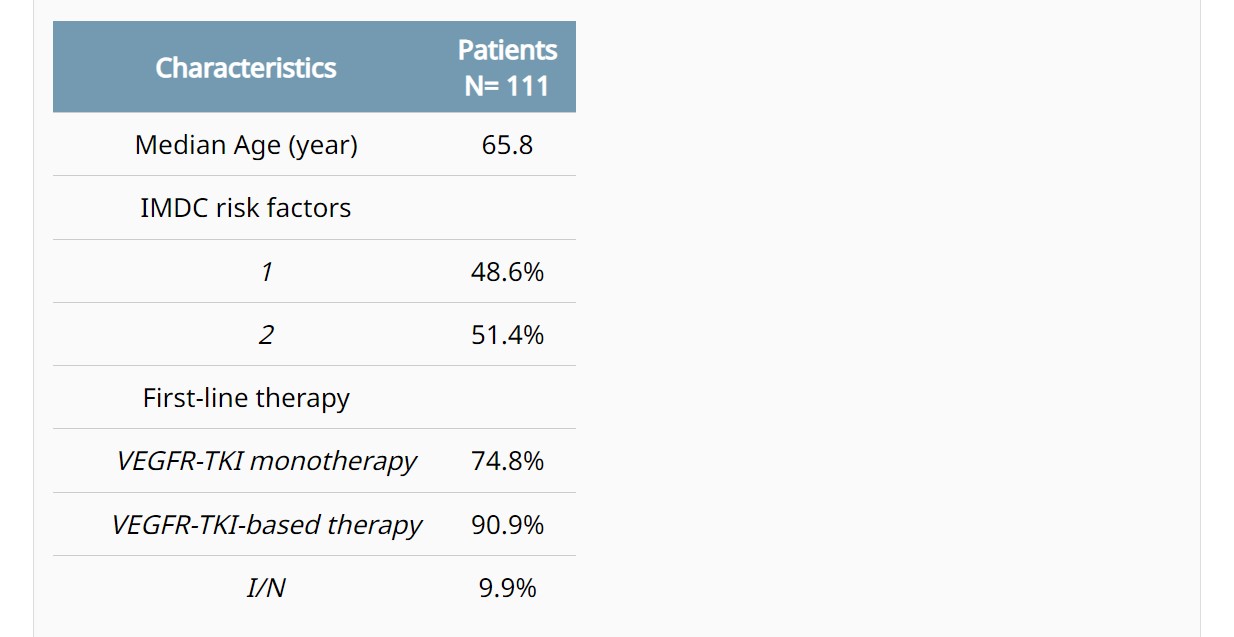
Background: Intermediate IMDC group is the largest and most heterogeneous group of mRCC. Current first-line (1L) therapy options for these patients (pts) are based on either an anti-angiogenic agent (VEGFR-TKI) combined with immunotherapy (IO), or a combo of IO (ipilimumab+nivolumab [I/N]). No biomarkers (BM) for selecting the most effective regimen have been identified so far. Methods: Immunohistochemical expression of PBRM1, PD-L1, CD31, and CD4/CD8 ratio was evaluated on histological samples of intermediate-risk mRCC pts treated with VEGFR-TKI monotherapy, and then in pts receiving a VEGFR-TKI-based therapy or the immune doublet I/N. PBRM1 positivity score was based on the percentage of positive cells and on the intensity of nuclear expression; PD-L1 positivity was defined as CPS≥10; CD31 high-density had moderate to strong nuclear staining; and the CD4/CD8 ratio cut-off for positivity was >0.2. Cox model was used to assess the correlation between BM and outcomes; PFS and OS were estimated by Kaplan-Meier method. Results: After screening of tumor tissues from 150 pts, a total of 111 were included in the final analysis (Table). In pts treated with VEGFR-TKI monotherapy, a significant correlation with PFS was observed with loss of PBRM1 expression (HR 0.58, p=0.035), PD-L1 negativity (HR 0.44, p=0.048), and high CD4/CD8 ratio (HR 0.62, p=0.073). CD31 density did not significantly correlate with PFS. A profile potentially predictive of angiogenesis (AP+) was defined based on the PBRM1 loss, PD-L1 negative, and high CD4/CD8. In pts treated with VEGFR-TKI monotherapy, tumors with the AP+ (43% of all cases) had a significantly longer median PFS (mPFS 23.8 vs. 11.8 months, p=0.003) and mOS (41.5 vs. 26.9 months, p=0.024) compared to the others. The AP+ retained its significant correlation with PFS (mPFS 23.8 vs. 11.1 months, p<0.001) and OS (41.5 vs. 24.9, p=0.006) in pts receiving VEGFR-TKI-based therapies. The rate of AP+ tumors was 55.6% and 32.7% in pts with one or two IMDC risk factors, respectively (p=0.022). In the small cohort of pts treated with I/N, no differences were observed in PFS (p=0.64) and OS (p=0.75) between AP+ and AP-negative. Conclusions: The AP+ signature (loss of PBRM1, PD-L1 negative, and CD4/CD8 high ratio) was associated with improved clinical outcomes in mRCC pts at IMDC intermediate prognosis treated with VEGFR-TKI-based therapy; this correlation was significant regardless from the addition of IO to VEGFR-TKI monotherapy. Prospective validation of this signature is required for guiding the selection of the most appropriate 1L therapy.


ABSTRACT TPS747
- -LITESPARK-024: A randomized phase 1/2 study of belzutifan with or without palbociclib in patients with advanced renal cell carcinoma.David F. McDermott, et al.
Background: The combination of immunotherapy with antiangiogenic agents is a well-established first-line treatment option for patients (pts) with advanced renal cell carcinoma (RCC), but many pts develop resistance, and effective second- or subsequent-line options are needed. The von Hippel-Lindau (VHL) gene is inactivated in approximately 90% of RCC cases, which results in the constitutive activation of hypoxia-inducible factor 2α (HIF-2α) signaling. HIF-2α is involved in angiogenesis, tumor growth, proliferation, and metastasis, and is a key oncogenic driver in RCC. The HIF-2α inhibitor belzutifan has demonstrated promising antitumor activity with manageable safety in pts with heavily pretreated RCC. The cyclin-dependent kinase (CDK) pathway is altered in several cancer types, including RCC, and is associated with poor clinical outcomes. The CDK 4/6 inhibitor palbociclib inhibited cell growth in RCC cell lines, and the antiproliferative effects of CDK 4/6 inhibition were synergistic with HIF-2α inhibition in HIF-2α–dependent VHL -/- clear cell RCC cell lines. We hypothesized palbociclib could potentially enhance the efficacy of belzutifan as combination therapy for previously treated pts with advanced RCC. Methods: LITESPARK-024 (NCT05468697) is an open-label, multicenter, phase 1/2 randomized study of belzutifan + palbociclib versus belzutifan monotherapy in pts with advanced RCC. Pts must have histologically confirmed unresectable stage IV RCC with a clear cell component, received at least 2 prior systemic regimens (both an anti–PD-1/PD-L1 monoclonal antibody and a VEGF receptor–targeted TKI, in sequence or in combination), have measurable disease per RECIST v1.1 by BICR, have KPS score of ≥70%, and have radiographic disease progression on or after the most recent regimen per investigator. Part 1 will evaluate the safety of belzutifan + palbociclib and determine the recommended phase 2 dose (RP2D) for the combination using a modified toxicity probability interval design. Part 2 will evaluate the safety and efficacy of belzutifan + palbociclib versus belzutifan alone. In part 1, ≤30 pts will be enrolled into 3 dose groups and receive belzutifan 120 mg once daily + palbociclib (75, 100, or 125 mg) daily for 21 consecutive days followed by 7 days off. In part 2, approximately 150 pts will be randomly assigned 2:1 to receive belzutifan 120 mg once daily + palbociclib RP2D (21 consecutive days/7 days off) or belzutifan 120 mg once daily. Pts will be stratified by IMDC risk (0 vs 1-2 vs 3-6) and sarcomatoid histology (yes vs no) at randomization in part 2. The primary end point for part 1 is to assess dose-limiting toxicities and adverse events to determine the RP2D of belzutifan + palbociclib. The primary end point for part 2 is ORR per RECIST v1.1 by investigator assessment. Secondary end points for part 2 are clinical benefit rate, DOR, PFS, OS, and safety and tolerability. Clinical trial information: NCT05468697.
ABSTRACT TPS748
- - Phase 3 LITESPARK-022: Pembrolizumab (pembro) plus hypoxia-inducible factor 2α (HIF-2α) inhibitor belzutifan as adjuvant treatment for clear cell renal cell carcinoma (ccRCC).Toni K. Choueiri, et al.
Background: Treatment with the PD-1 inhibitor pembro produced significant improvement in disease-free survival after surgery for patients (pts) with ccRCC in the phase 3 KEYNOTE-564 trial. Based on these results, pembro was approved by the US Food and Drug Administration and the European Medicines Agency for adjuvant treatment of pts with RCC at increased risk of recurrence following nephrectomy or following nephrectomy and resection of metastatic lesions. Despite advances in the treatment landscape for RCC, more effective adjuvant treatment strategies are needed for pts at risk of recurrence after surgery. HIF-2α is an established oncogenic driver in ccRCC, and promising antitumor activity in advanced ccRCC and von Hippel-Lindau disease–associated RCC has been demonstrated with the HIF-2α inhibitor belzutifan. The multicenter, double-blind, randomized, phase 3 LITESPARK-022 study (NCT05239728) will evaluate the efficacy and safety of pembro plus belzutifan compared with placebo plus pembro as adjuvant treatment following nephrectomy in pts with ccRCC. Methods: Key eligibility criteria include adults with histologically or cytologically confirmed intermediate-high risk, high risk, or M1 with no evidence of disease (NED) RCC with a clear cell component; pts with no prior systemic therapy, nephrectomy, and/or metastasectomy ≤12 weeks before randomization; and pts who are tumor free per computed tomography/magnetic resonance imaging. Approximately 1600 pts will be randomly assigned to receive belzutifan 120 mg orally once daily plus pembro 400 mg intravenously (IV) every 6 weeks (Q6W) for ≤9 administrations (~54 weeks) or oral placebo plus pembro 400 mg IV Q6W for ≤9 administrations (~54 weeks) or until verified disease recurrence by blinded independent central review, start of new anticancer treatment, unacceptable toxicity, or decision to withdraw. Stratification factors are tumor grade (1 or 2 vs 3 or 4) and risk type (intermediate-high risk versus high risk versus M1 NED). Pts will be radiologically evaluated Q12W from randomization through year 2, Q16W in years 3 to 5, and Q24W in years 6 and beyond. Adverse events will be monitored throughout the study and for 30 days following cessation of study treatment (90 days for serious adverse events). The primary end point is disease-free survival. Secondary end points include overall survival, safety, disease recurrence–specific survival, and patient-reported outcomes. Recruitment is underway in Asia, Australia, Europe, North America, and South America. © 2022 American Society of Clinical Oncology, Inc. Reused with permission. This abstract was accepted and previously presented at the 2022 ASCO Annual Meeting. All rights reserved. Clinical trial information: NCT05239728.