ASCO Abstracts:
![]() Download PDF
Download PDF
RECOMMENDED ABSTRACTS in RENAL CANCERS

 Some Other Text
Some Other Text

These recommended abstracts from ASCO23 Annual meeting have been selected by Robert A. Figlin, MD, Editor-in- Chief of the Kidney Cancer Journal. The chosen abstracts provided here highlight some of the most important trends in ongoing trials and reflect the foremost research and strategies from latest clinical trials that impact the current standard of care in renal cancer.





ABSTRACT LBA 4500
Efficacy and safety of atezolizumab plus cabozantinib vs cabozantinib alone after progression with prior immune checkpoint inhibitor (ICI) treatment in metastatic renal cell carcinoma (RCC): Primary PFS analysis from the phase 3, randomized, open-label CONTACT-03 study.
Toni K. Choueiri, Laurence Albiges, Piotr Tomczak, Cristina Suárez, Martin H. Voss, Guillermo de Velasco, Jad Chahoud, Giuseppe Procopio, Hakim Mahammedi, Friedemann Zengerling, Chan Kim, Suyasha Gupta, Guillaume Bergthold, Bo Liu, Melania Kalaitzidou, Mahrukh A. Huseni, Christian Scheffold, Thomas Powles, Sumanta Kumar Pal
ORGANIZATIONS
Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, Medical Oncology, Gustave Roussy, Université Paris Saclay, Paris, France, Clinical Hospital No. 1 of the Poznan University of Medical Sciences, Poznań, Poland, Vall d'Hebron University Hospital, Barcelona, Spain, Memorial Sloan Kettering Cancer Center and Weill Medical College, New York, NY, Medical Oncology Department, Hospital Universitario 12 de Octubre, Madrid, Spain, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy, Jean Perrin Cancer Center, Clermont-Ferrand, France, Department of Urology und Paediatric Urology, Hospital University of Ulm, Ulm, Germany, Medical Oncology, CHA Bundang Medical Center, CHA University School of Medicine, Korea, Bundang-Gu, Seongnam-Si, South Korea, Genentech Inc, South San Francisco, CA, F. Hoffmann-La Roche Ltd, Basel, Switzerland, Roche, Muswell Hill, United Kingdom, Exelixis, Inc., Alameda, CA, Barts Cancer Institute, Experimental Cancer Medicine Centre, Queen Mary University of London, St. Bartholomew's Hospital, London, United Kingdom, Department of Medical Oncology & Therapeutics, City of Hope Comprehensive Cancer Center, Duarte, CA
BACKGROUND ICI-based regimens are the standard of care for first-line (1L) treatment of metastatic clear cell (cc) RCC. Treatment options following disease progression during or after ICI therapy are limited but can include single-agent TKIs, such as cabozantinib (cabo). CONTACT-03 evaluated anti–PD-L1 atezolizumab (atezo) + cabo vs cabo alone in patients (pts) with metastatic RCC that progressed during or after prior ICI treatment and is the first phase 3 randomized trial to test the benefit of ICI rechallenge by direct addition to a control arm.
METHODS: CONTACT-03 enrolled pts with histologically confirmed, inoperable, locally advanced or metastatic cc or non-cc RCC, regardless of PD-L1 status, that progressed on or after ICI treatment. Randomization was 1:1 to atezo (1200 mg IV q3w) plus cabo (60 mg oral qd) or cabo alone. Stratification factors were IMDC risk factors (0 vs 1-2 vs ≥3); most recent line of prior ICI therapy (adjuvant vs 1L vs 2L); and histology (dominant cc without sarcomatoid vs dominant non-cc [papillary or unclassified] without sarcomatoid vs cc or non-cc with any sarcomatoid component). The multiple primary efficacy endpoints were centrally reviewed RECIST 1.1 PFS and OS. Key secondary endpoints were investigator (INV)-assessed PFS, centrally reviewed RECIST 1.1 ORR and DOR and safety.
RESULTS: Of 522 pts randomized to atezo + cabo (n=263) or cabo (n=259), 55% and 51% had most recent ICI in the 1L setting and 10% and 11% had sarcomatoid RCC, respectively. At the data cutoff (Jan 3, 2023), median follow-up was 15.2 mo. No PFS or OS benefit was observed with atezo + cabo vs cabo. ORR was 41% in both arms; DOR was 12.7 (95% CI: 10.5, 17.4) mo with atezo + cabo and 14.8 (95% CI: 11.3, 20.0) mo with cabo. All-cause Grade 3/4 adverse events (AEs) occurred in 68% (177/262) and 62% (158/256) of safety-evaluable pts receiving atezo + cabo or cabo, respectively; all-cause Grade 5 AEs occurred in 6% and 4%. AEs leading to treatment withdrawal occurred in 16% of pts on atezo + cabo and 4% on cabo.atment-emergent adverse events (TEAEs), 2 TEAEs were treatment related.
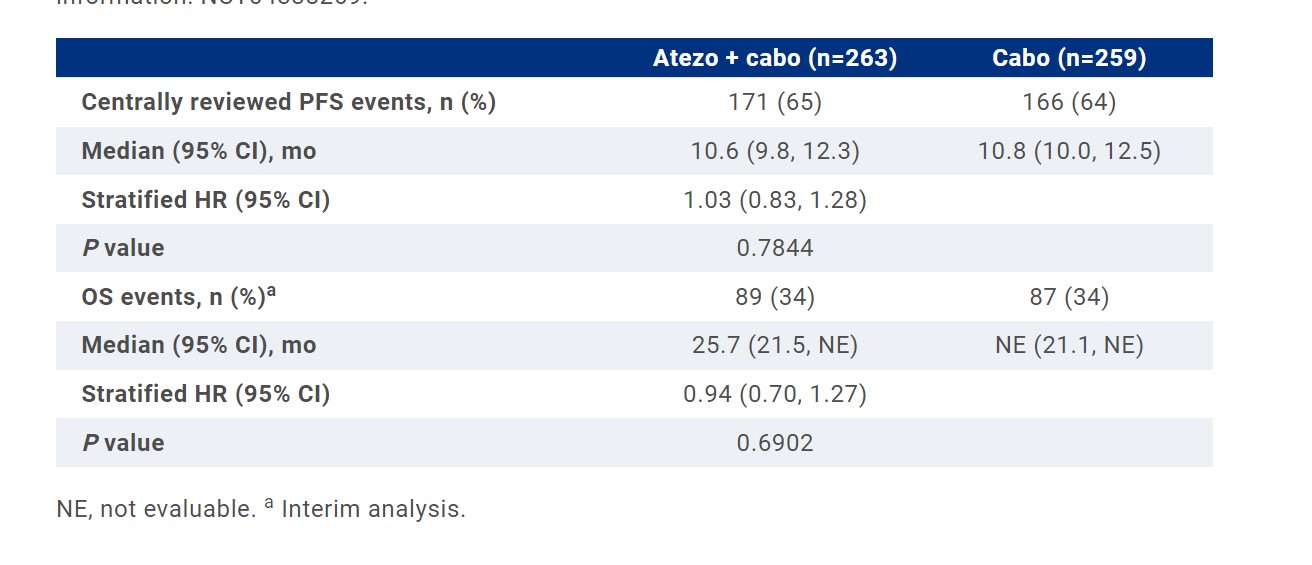 CONCLUSIONS:
The addition of atezo to cabo did not improve clinical outcomes and led to increased toxicity in patients with RCC that progressed on or after prior ICI treatment. CONTACT-03 is the first randomized, phase III oncology trial to test the benefit of PD-(L)1 inhibitor continuation by direct addition to a standard control arm; the results prompt caution with this approach in other cancers. Clinical trial information: NCT04338269.
CONCLUSIONS:
The addition of atezo to cabo did not improve clinical outcomes and led to increased toxicity in patients with RCC that progressed on or after prior ICI treatment. CONTACT-03 is the first randomized, phase III oncology trial to test the benefit of PD-(L)1 inhibitor continuation by direct addition to a standard control arm; the results prompt caution with this approach in other cancers. Clinical trial information: NCT04338269.
ABSTRACT LBA 4501
- Pembrolizumab plus axitinib versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma: 5-year analysis of KEYNOTE-426.
Brian I. Rini, Elizabeth R. Plimack, Viktor Stus, Rustem Gafanov, Tom Waddell, Dmitry Nosov, Frederic Pouliot, Boris Alekseev, Denis Soulieres, Bohuslav Melichar, Ihor O. Vynnychenko, Sergio Jobim Azevedo, Delphine Borchiellini, Raymond S. McDermott, Jens Bedke, Satoshi Tamada, Sterling Wu, Joseph Burgents, L. Rhoda Molife, Thomas Powles
ORGANIZATIONS
Vanderbilt-Ingram Cancer Center, Nashville, TN, Fox Chase Cancer Center, Philadelphia, PA, Dnipro State Medical University, Dnipro, Ukraine, Russian Scientific Center of Roentgenoradiology, Moscow, Russian Federation, Christie Hospital, Manchester, United Kingdom, Central Clinical Hospital With Outpatient Clinic, Moscow, Russian Federation, CHU of Quebec and Laval University, Quebec, QC, Canada, P. A. Herzen Moscow Oncology Research Institute, Ministry of Health of the Russian Federation, Moscow, Russian Federation, Centre Hospitalier de l’Université de Montréal, Montreal, QC, Canada, Palacky University Medical School, Olomouc, Czech Republic, Sumy State University, Sumy, Ukraine, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil, Centre Antoine Lacassagne, Université Côte d’Azur, Nice, France, Adelaide and Meath Hospital, University College Dublin, Dublin, Ireland, Eberhard Karls University of Tübingen, Tübingen, Germany, Bell-Land General Hospital, Osaka, Japan, Merck & Co., Inc., Rahway, NJ, Barts Health NHS Trust and the Royal Free NHS Foundation Trust, Barts Cancer Institute, and Queen Mary University of London, London, United Kingdom
BACKGROUND At the first interim analysis of the randomized, open-label, phase 3 KEYNOTE-426 (NCT02853331) study, 1L pembro + axi showed statistically significant OS, PFS, and ORR over sun for advanced ccRCC. We report results with 5-y minimum follow-up.
METHODS: Adults with confirmed locally advanced or metastatic ccRCC with or without sarcomatoid features, no previous systemic therapy for metastatic ccRCC, KPS ≥70%, and ≥1 lesion measurable per RECIST v1.1 were randomly assigned 1:1 to receive pembro 200 mg IV Q3W for 35 doses (~2 y) + axi 5 mg PO BID or sun 50 mg PO QD on a 4-wk-on/2-wk-off schedule. Dual primary end points were OS and PFS per RECIST v1.1 by blinded independent central review (BICR). Secondary end points included ORR and DOR per RECIST v1.1 by BICR, and safety. A post hoc analysis adjusting for the effect of subsequent therapy on OS using a 2-stage adjustment model was conducted.
RESULTS: Of 861 enrolled patients (pts), 432 were assigned to pembro + axi and 429 to sun. Median study follow-up was 67.2 mo (range, 60.0-75.0). Efficacy for the ITT population and IMDC risk subgroups are shown in table. For pembro + axi vs sun, the 60-mo OS rates were 41.9% vs 37.1%, and the 60-mo PFS rates were 18.3% vs 7.3%. Median DOR (range) was 23.6 mo (1.4+ to 68.6+) for pembro + axi and 15.3 mo (2.3-68.3) for sun. In pts who discontinued treatment, 237/381 pts (62.2%) in the pembro + axi arm and 300/406 pts (73.9%) in the sun arm received subsequent anticancer treatment. The HR for OS when adjusted for subsequent therapy was 0.67 (95% CI, 0.52-0.84). Clinical data on pts who completed 2 y of pembro will be presented. No new safety signals were observed.
CONCLUSIONS: After 5 y of follow-up, pembro + axi had sustained OS, PFS, and ORR benefits over sun in advanced ccRCC. These results are the longest follow-up to date of an anti–PD-1/L1 inhibitor + VEGFR TKI in this pt population and continue to support the use of pembro + axi as a 1L standard of care for advanced ccRCC.
Clinical trial information: NCT02853331.
ABSTRACT 4502
- Final prespecified overall survival (OS) analysis of CLEAR: 4-year follow-up of lenvatinib plus pembrolizumab (L+P) vs sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC).
Robert J. Motzer, Camillo Porta, Masatoshi Eto, Thomas Powles, Viktor Grünwald, Thomas E. Hutson, Boris Alekseev, Sun Young Rha, Jaime R. Merchan, Jeffrey C. Goh, Anil Kapoor, Ugo De Giorgi, Bohuslav Melichar, Sung-Hoo Hong, Howard Gurney, Karla Rodriguez-Lopez, Cixin S. He, Chinyere Okpara, Jodi McKenzie, Toni K. Choueiri
ORGANIZATIONS
Memorial Sloan Kettering Cancer Center, New York, NY, University of Bari 'A. Moro', Bari, Italy, Kyushu University, Fukuoka, Japan, The Royal Free NHS Trust, London, United Kingdom, University Hospital Essen, Essen, Germany, Texas Oncology, Dallas, TX, P.A. Herzen Moscow Oncological Research Institute, Moscow, Russian Federation, Yonsei Cancer Center, Yonsei University Health System, Seoul, Korea, Republic of (South), University of Miami Sylvester Comprehensive Cancer Center, Miami, FL, ICON Research, South Brisbane and Queensland University of Technology, Brisbane, QLD, Australia, Juravinski Cancer Centre, McMaster University, Hamilton, ON, Canada, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) Dino Amadori, Meldola, Italy, Palacký University Medical School and Teaching Hospital, Olomouc, Czech Republic, Seoul St. Mary's Hospital, Seoul, South Korea, Macquarie University, Sydney, NSW, Australia, Merck & Co., Inc., Rahway, NJ, Eisai Inc., Nutley, NJ, Eisai Ltd., Hatfield, United Kingdom, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA
BACKGROUND In the phase 3 CLEAR trial, L+P showed clinically meaningful and statistically significant benefits in PFS (primary endpoint) and OS, and improved ORR compared with S in 1L aRCC (Motzer NEJM 2021). Here, we report 4-yr follow-up results from the final prespecified OS analysis of CLEAR (data cutoff: 31 Jul 2022).
METHODS: Treatment-naïve pts (n=1069) who had aRCC with a clear-cell component were randomized (1:1:1) to receive: L 20 mg PO QD + P 200 mg IV Q3W; or L 18 mg + everolimus 5 mg PO QD; or S 50 mg PO QD (4 wks on/2 wks off). Stratification factors were geographic region and MSKCC prognostic risk group. This final prespecified OS analysis was triggered by ~304 death events in 2 arms. OS, PFS, ORR, duration of response (DOR), and PFS on next-line therapy (PFS2) were assessed for L+P and S. PFS, ORR and DOR were assessed per independent review using RECIST v1.1. Nominal P-values are shown.
RESULTS: At a median follow-up (IQR) of 49.8 mos (41.4–53.1) for L+P and 49.4 mos (41.6–52.8) for S, 149 and 159 deaths had occurred, respectively. OS benefit with L+P vs S was maintained (HR, 95% CI; 0.79, 0.63–0.99). OS favored L+P vs S across MSKCC risk groups (HR, 95% CI; favorable [fav]: 0.89, 0.53–1.50; intermediate [int]: 0.81, 0.62–1.06; poor: 0.59, 0.31–1.12). PFS benefit of L+P vs S was maintained (HR, 95% CI; 0.47, 0.38–0.57). PFS favored L+P vs S across MSKCC risk groups (HR, 95% CI; fav: 0.46, 0.32–0.67; int: 0.51, 0.40–0.65; poor: 0.18, 0.08–0.42). ORR was greater with L+P (71.3%; complete response [CR], 18.3%) vs S (36.7%; CR, 4.8%) (relative risk, 95% CI; 1.94, 1.67–2.26). Less pts in the L+P arm (181/355, 51.0%) received subsequent anticancer therapies compared with the S arm (246/357, 68.9%); 56 (15.8%) and 195 (54.6%) received PD-1/PD-L1 checkpoint inhibitors, respectively. Analysis of OS adjusted for subsequent therapies will be presented. PFS2 was longer with L+P vs S (43.3 vs 25.9 mos; HR, 95% CI; 0.63, 0.51–0.77). Grade ≥3 treatment-related adverse events occurred in 74.1% and 60.3% pts in the L+P and S arms, respectively.
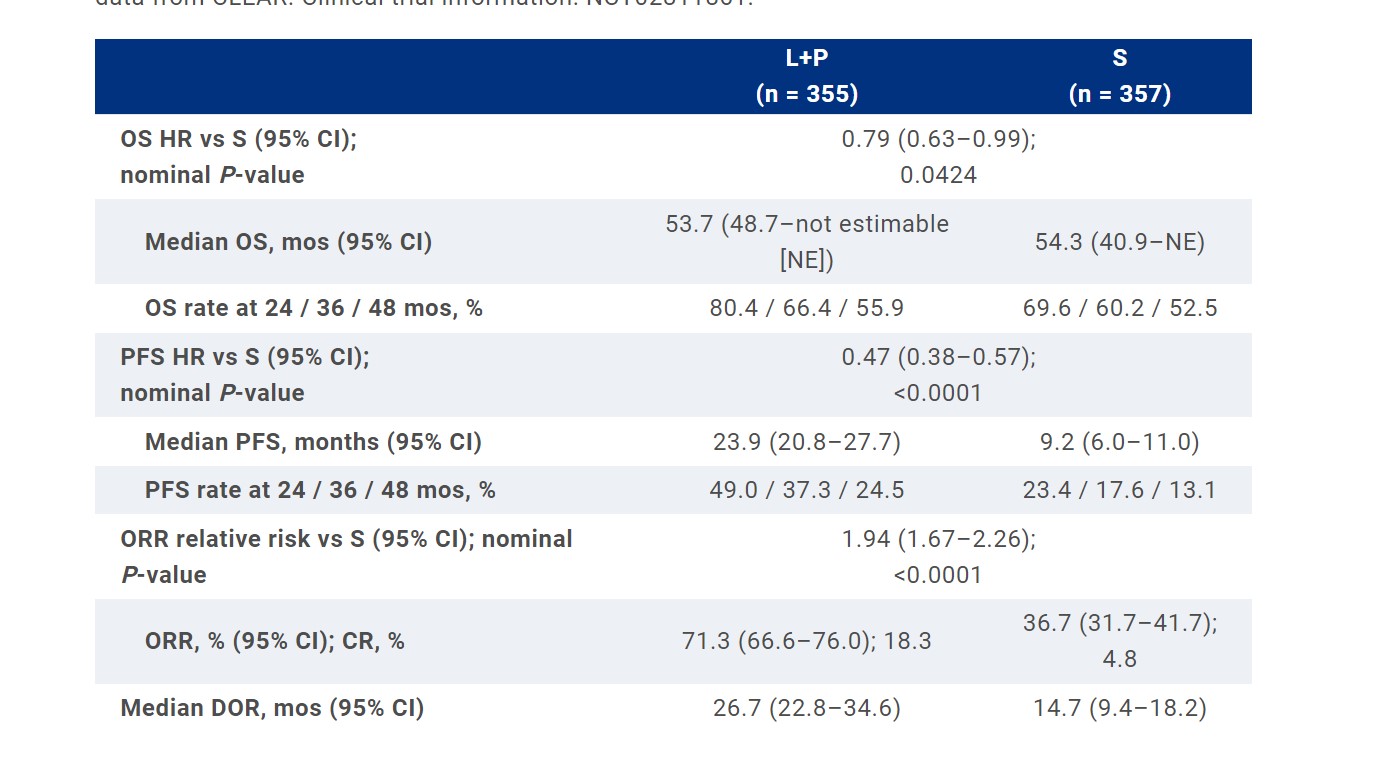 CONCLUSIONS:
L+P continues to demonstrate clinically meaningful benefit vs S in OS, PFS, ORR, and CR in the 1L treatment of pts with aRCC at 4-yr follow-up, thus supporting the robustness of the primary analysis data from CLEAR. Clinical trial information: NCT02811861.
CONCLUSIONS:
L+P continues to demonstrate clinically meaningful benefit vs S in OS, PFS, ORR, and CR in the 1L treatment of pts with aRCC at 4-yr follow-up, thus supporting the robustness of the primary analysis data from CLEAR. Clinical trial information: NCT02811861.
ABSTRACT 4506
- Adjuvant nivolumab plus ipilimumab vs placebo for patients with localized renal cell carcinoma at high risk of relapse after nephrectomy: Subgroup analyses from the phase 3 CheckMate 914 (part A) trial.
Robert J. Motzer, Paul Russo, Viktor Grünwald, Yoshihiko Tomita, Philippe Barthélémy, Jeffrey C. Goh, Hernán Javier Cutuli, Burcin Simsek, Julia Spiridigliozzi, Aleksander Chudnovsky, Axel Bex
ORGANIZATIONS
Memorial Sloan Kettering Cancer Center, New York, NY, Clinic for Internal Medicine (Tumor Research) and Clinic for Urology, West-German Cancer Center Essen, University Hospital Essen, Essen, Germany, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan, Institut de Cancérologie Strasbourg Europe, Strasbourg, France, ICON Research, South Brisbane, QLD, Australia, Sirio Libanes Hospital, Buenos Aires, Argentina, Bristol Myers Squibb, Princeton, NJ, Netherlands Cancer Institute, Amsterdam, Netherlands
BACKGROUND In part A of the CheckMate 914 trial, adjuvant nivolumab plus ipilimumab (NIVO+IPI) did not improve disease-free survival (DFS) vs placebo (PBO) in patients (pts) with localized renal cell carcinoma (RCC) at high risk of post-nephrectomy relapse (Motzer RJ, et al. Lancet 2023). Exploratory analyses were conducted to better understand outcomes in key pt subsets and with early NIVO+IPI discontinuation.
METHODS: Key study inclusion criteria were radical/partial nephrectomy with negative margins > 4 and ≤ 12 weeks before randomization; predominant clear cell histology; pathological TNM stage T2a (grade [G] 3/4) N0M0, T2b-T4 (any G) N0M0, or any pT (any G) N1M0; and no evidence of residual disease/metastases. Pts in part A were randomized 1:1 to NIVO 240 mg Q2W (× 12) + IPI 1 mg/kg Q6W (× 4) or equivalent PBO for 24 weeks or until recurrence/unacceptable toxicity. Primary endpoint is DFS per blinded independent central review. Exploratory analyses assessed DFS by key subsets including Fuhrman grade, sarcomatoid features (yes/no), PD-L1 expression, and NIVO+IPI exposure (≤ 6 cycles [1–2 IPI doses] vs > 6 cycles [3–4 IPI doses]). Safety was assessed by exposure.
RESULTS: 816 pts were randomized to adjuvant NIVO+IPI (N = 405) or PBO (N = 411). At 37.0 months median follow-up (min, 15.4 months), subset analyses suggested a DFS benefit for NIVO+IPI vs PBO in pts with Fuhrman grade 4 or sarcomatoid features. DFS by PD-L1 expression will be reported in the presentation. Pts who received > 6 NIVO+IPI cycles trended toward improved DFS vs pts receiving ≤ 6 NIVO+IPI cycles. Of the 102 pts who received ≤ 6 NIVO+IPI cycles, 3% had sarcomatoid features, and 20% had Fuhrman grade 4; treatment discontinuation in these pts was due to study drug toxicity (75%), unrelated adverse events (AEs; 6%), pt request (5%), recurrence (4%), consent withdrawal/non-compliance (4%), or other (6%), and most pts receiving ≤ 6 NIVO+IPI cycles were discontinued without initial dose delay (NIVO, 84%; IPI, 89%). In the group of patients who received ≤ 6 NIVO+IPI cycles, grade 1–2 all-cause AEs were reported in 35% of pts (grade ≥ 3, 63%) and 31% of pts discontinued treatment due to grade 1–2 all-cause AEs (grade ≥ 3, 44%).
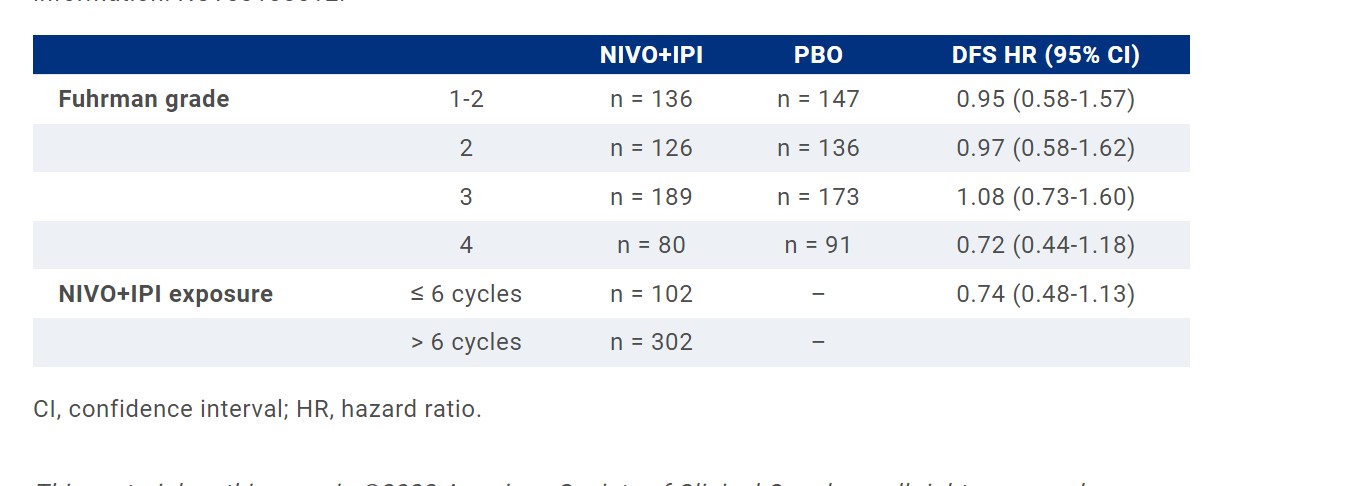 CONCLUSIONS:
Exploratory analyses suggest that tumor grade and sarcomatoid features influence outcomes with adjuvant NIVO+IPI. Limited NIVO+IPI exposure (≤ 6 cycles) and discontinuation for low-grade AEs may have contributed to the lack of DFS benefit observed in CheckMate 914 part A. Clinical trial information: NCT03138512.
CONCLUSIONS:
Exploratory analyses suggest that tumor grade and sarcomatoid features influence outcomes with adjuvant NIVO+IPI. Limited NIVO+IPI exposure (≤ 6 cycles) and discontinuation for low-grade AEs may have contributed to the lack of DFS benefit observed in CheckMate 914 part A. Clinical trial information: NCT03138512.
ABSTRACT 4518
- First-line lenvatinib + pembrolizumab treatment across non-clear cell renal cell carcinomas: Results of the phase 2 KEYNOTE-B61 study.
Chung-Han Lee, Howard Gurney, Vagif Atduev, Cristina Suárez, Miguel A Climent Duran, David William Pook, Piotr Tomczak, Philippe Barthelemy, Jae-Lyun Lee, Taron Nalbandian, Viktor Stus, Thomas Ferguson, Pawel Wiechno, Erhan Gokmen, Louis Lacombe, Craig Gedye, Joseph Burgents, Manish Sharma, Xiang Peng, Laurence Albiges
ORGANIZATIONS
Memorial Sloan Kettering Cancer Center, New York, NY, Macquarie University, Sydney, NSW, Australia, Volga District Medical Center, Federal Medical-Biological Agency, Nizhny Novgorod, Russian Federation, Medical Oncology, Vall d´Hebron Institute of Oncology (VHIO), Hospital Universitari Vall d´Hebron, Vall d´Hebron Barcelona Hospital Campus, Barcelona, Spain, Instituto Valenciano de Oncología, València, Spain, Monash Health, Melbourne, VIC, Australia, Poznan University of Medical Sciences, Poznan, Poland, Institut de Cancérologie Strasbourg Europe, Strasbourg, France, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea, Regional Cancer Center, Kharkiv, Kharkiv, Ukraine, Dnipro State Medical University, Dnipro, Ukraine, Fiona Stanley Hospital, Perth, Western Australia, Australia, Oncology Center-Institute Marii Sklodowskiej-Curie, Warsaw, Poland, Ege University Medical Faculty, Izmir, Turkey, Centre de Recherche du CHU de Québec, Québec City, QC, Canada, University of Newcastle, Callaghan, NSW, Australia, Merck & Co., Inc., Rahway, NJ, Gustave Roussy, Villejuif, France
BACKGROUND Lenvatinib (lenva) + pembrolizumab (pembro) is a first-line treatment for advanced clear cell renal cell carcinoma (RCC). Initial results of the single-arm, phase 2 KEYNOTE-B61 (NCT04704219) study showed antitumor activity of lenva + pembro in patients (pts) with advanced non-clear cell RCC who had opportunity for ≥24 wk of follow-up (n=82). We report results from the complete cohort of pts enrolled in KEYNOTE-B61 (N=158) with extended follow-up.
METHODS: Adults with previously untreated advanced non-clear cell RCC and measurable disease per RECIST v1.1 received lenva 20 mg PO QD + pembro 400 mg IV Q6W for up to 18 cycles (~2 y). The primary end point was ORR per RECIST v1.1 by blinded independent central review (BICR). Secondary end points included DOR, DCR, and PFS per RECIST v1.1 by BICR; OS; and safety. Histology was assessed by investigator (assessment by central review is planned).
RESULTS: Of 158 treated pts, 93 (59%), 29 (18%), and 21 (13%) had papillary, chromophobe, and unclassified histology, respectively. Additionally, 6 pts (4%) had translocation and 9 (6%) had other histology. 70 pts (44%) had IMDC favorable risk and 88 (56%) had intermediate/poor risk. Median follow-up was 14.9 mo (range 8.7-19.7). ORR was 49% (95% CI, 41-57; 9 CRs [6%]; 69 PRs [44%]). DCR was 82% (95% CI, 75-88). Median DOR was not reached (NR; range, 1.5+ to 15.3+ mo). By Kaplan-Meier estimate, 75% of responders had a response for ≥12 mo. ORR and DCR by histology are shown in the table. For the IMDC favorable risk group, ORR was 51% (95% CI, 39-64) and DCR was 87% (95% CI, 77-94). For the IMDC intermediate/poor risk group, ORR was 48% (95% CI, 37-59) and DCR was 78% (95% CI, 68-86). In all pts, median PFS and OS were 17.9 mo (95% CI, 13.5-NR) and NR (95% CI, NR-NR), respectively; 12-mo rates were 63% and 82%. Treatment-related AEs (TRAEs) occurred in 149 pts (94%) and were consistent with results from other studies. The most common (≥30%) TRAEs were hypertension (n=90; 57%), diarrhea (n=69; 44%), and hypothyroidism (n=58; 37%). Grade 3-4 TRAEs occurred in 81 pts (51%). Overall, 17 pts (11%) discontinued pembro, 14 (9%) discontinued lenva, and 5 (3%) discontinued both drugs because of TRAEs. No deaths occurred because of TRAEs.
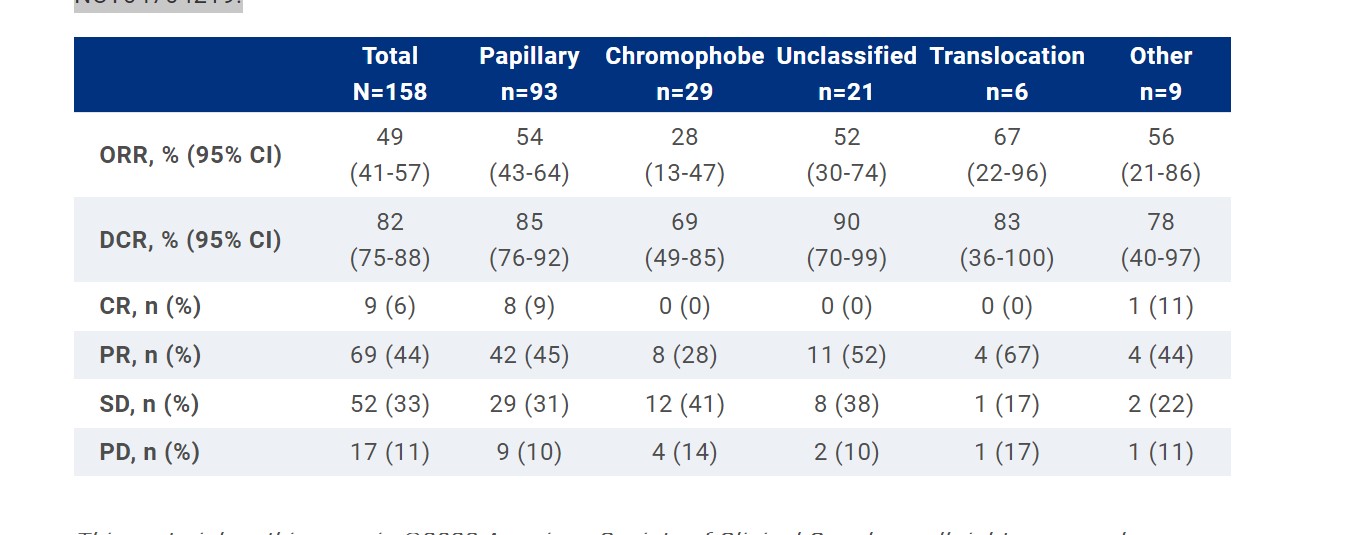
CONCLUSIONS:
In pts with advanced non-clear cell RCC enrolled in KEYNOTE-B61, lenva + pembro showed antitumor activity with no new safety signals. These data support the use of lenva + pembro as first-line treatment for pts with non-clear cell RCC, regardless of histology. Clinical trial information: NCT04704219.
ABSTRACT 4519
- Efficacy of first-line (1L) immunotherapy (IO)-based regimens in patients with sarcomatoid and/or rhabdoid (S/R) metastatic non-clear cell renal cell carcinoma (nccRCC): Results from the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC).
Chris Labaki, Ziad Bakouny, Audreylie Lemelin, Matthew Scott Ernst, Kosuke Takemura, J Connor Wells, Sumanta Kumar Pal, Bernadett Szabados, Benoit Beuselinck, Rana R. McKay, Jae-Lyun Lee, Takeshi Yuasa, Francis Parnis, Georg A. Bjarnason, Bradley Alexander McGregor, David A. Braun, Wanling Xie, Wenxin Xu, Daniel Yick Chin Heng, Toni K. Choueiri
ORGANIZATIONS
The Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute and Brigham and Women's Hospital, Boston, MA, Tom Baker Cancer Centre, University of Calgary, Calgary, AB, Canada, BC Cancer Agency, Vancouver, Canada, Calgary, AB, Canada, City of Hope, Duarte, CA, Barts Cancer Institute, London, United Kingdom, UZ Gasthuisberg - Katholieke University Leuven, Leuven, Belgium, University of California San Diego, La Jolla, CA, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea, Cancer Institute Hospital, Japanese Foundation for Cancer Research, Tokyo, Japan, Adelaide Cancer Center, Kurralta Park, Australia, Sunnybrook Odette Cancer Centre, Toronto, ON, Canada, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, School of Medicine, Yale University, New Haven, CT, Department of Data Sciences, Dana-Farber Cancer Institute, Boston, MA, Dana-Farber Cancer Institute, Boston, MA
BACKGROUND Patients with advanced RCC with S/R components exhibit poor clinical outcomes. IO-based combination therapies demonstrated substantial efficacy among patients with metastatic S/R ccRCC, compared to VEGF targeted therapy (VEGF-TT). Recent trials showed promising activity of IO-based regimens in patients with advanced nccRCC. We sought to assess the efficacy of IO regimens among patients with S/R nccRCC.
METHODS: Patients with advanced nccRCC treated with 1L IO regimens (IO/IO or IO/VEGF-TT) or 1L VEGF-TT monotherapy (sunitinib or pazopanib) were included. Cases were categorized as S/R or non-S/R. The primary outcomes were overall survival (OS) and time to treatment failure (TTF) in patients with S/R nccRCC receiving 1L IO or VEGF-TT. Overall response rate (ORR) was a secondary outcome. OS and TTF were compared between groups (IO vs. VEGF-TT) using Cox regression models adjusted for age, IMDC risk groups, and nccRCC subtype. ORR was compared between groups (IO vs. VEGF-TT) using a logistic regression adjusted for the same confounders.
RESULTS: Overall, 103 patients with S/R nccRCC were included, of whom 33 (32%) received 1L IO regimens. Median follow-up was 31 months. After adjustment for confounding factors, patients with S/R nccRCC treated with IO regimens presented with significantly improved survival outcomes as compared to those receiving VEGF-TT (median OS [mOS]: NR vs. 7.1 and mTTF: 9.4 vs. 2.9 mos for IO regimens and VEGF-TT, respectively). Similarly, a higher ORR was seen in patients with S/R nccRCC receiving IO regimens versus VEGF-TT (34.1 vs. 10.9%, respectively). Among 430 patients with non-S/R nccRCC (IO regimens: n=44), no significant differences in survival outcomes between regimen classes were seen (mOS: 24.4 vs. 14.8 and mTTF: 4.2 vs. 5.0 mos for IO regimens and VEGF-TT, respectively).

CONCLUSIONS: To our knowledge, this represents the largest effort to characterize the outcomes of patients with S/R nccRCC treated with IO regimens. Patients with S/R nccRCC appear to derive a substantial and selective benefit from IO regimens (vs. VEGF-TT). These data support the use of IO-based regimens in patients with S/R nccRCC.
ABSTRACT 4520
- Phase II study of cabozantinib (Cabo) with nivolumab (Nivo) and ipilimumab (Ipi) in advanced renal cell carcinoma with variant histologies (RCCvh).
Bradley Alexander McGregor, Jiaming Huang, Wanling Xie, Wenxin Xu, Mehmet Asim Bilen, David A. Braun, Tian Zhang, Rana R. McKay, David F. McDermott, Hans J. Hammers, Toni K. Choueiri
ORGANIZATIONS
Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, Dana-Farber Cancer Institute, Boston, MA, Department of Data Sciences, Dana-Farber Cancer Institute, Boston, MA, Winship Cancer Institute of Emory University, Atlanta, GA, School of Medicine, Yale University, New Haven, CT, University of Texas Southwestern Medical Center, Dallas, TX, University of California San Diego, La Jolla, CA, Beth Israel Deaconess Medical Center, Boston, MA
BACKGROUND New therapeutic approaches in RCCvh are needed. Herein, we report on treatment intensification with the combination of Cabo/Nivo/Ipi in patients with metastatic RCCvh in a multi-institutional prospective single arm phase II trial. (NCT04413123).
METHODS: Eligible patients (pts) had metastatic RCCvh with ECOG performance status of 0-1 and may have received one line of prior therapy excluding immunotherapy or Cabo. Pts underwent a baseline biopsy and received treatment with Nivo 3 mg/kg and Ipi 1 mg/kg intravenously Q3 weeks (W) for 4 cycles followed by Nivo 480 mg IV Q4W. Cabo was given continuously at dose of 40 mg daily; reductions to 20 mg daily and 20 mg every other day were allowed. The primary endpoint was objective response rate (ORR) by RECIST 1.1. Safety was a secondary endpoint.
RESULTS: 40 pts have been enrolled. At the time of data cut-off (Dec 9, 2022), 38 pts received at least 1 study drug. 11% (n=4) pts received prior systemic therapy. 45% (n=17) received all 4 doses of Nivo and Ipi; 18% (n=7) received 3 and 37% (n=14) received ≤ 2 doses. 61% (n=23) (15 of whom received 4 cycles Nivo/Ipi) received Nivo maintenance (median number of cycles, 5 (range, 1-21)). 71% (n=27) and 13% (n=5) required Cabo dose reduction to 20 mg and 20 mg every other day, respectively. Median follow-up was 8.4 (range, 2.1-23) months. Objective response was achieved in 8 pts (ORR 21%, two-sided 80% CI, 13%-32%). Median duration of response was not reached with 5 pts maintaining response > 6 months. Median progression-free survival was 8.9 (95% CI, 4.2-12.7) months. 74% (n=28) developed treatment-related grade 3 or higher toxicities; 37% (n=14) developed ≥ grade 3 elevation in AST or ALT. 29% (n=11) required high dose steroids (prednisone ≥ 40 mg daily or equivalent). 13% (n=5) discontinued all study drugs due to toxicity. No grade 5 toxicity has been reported.
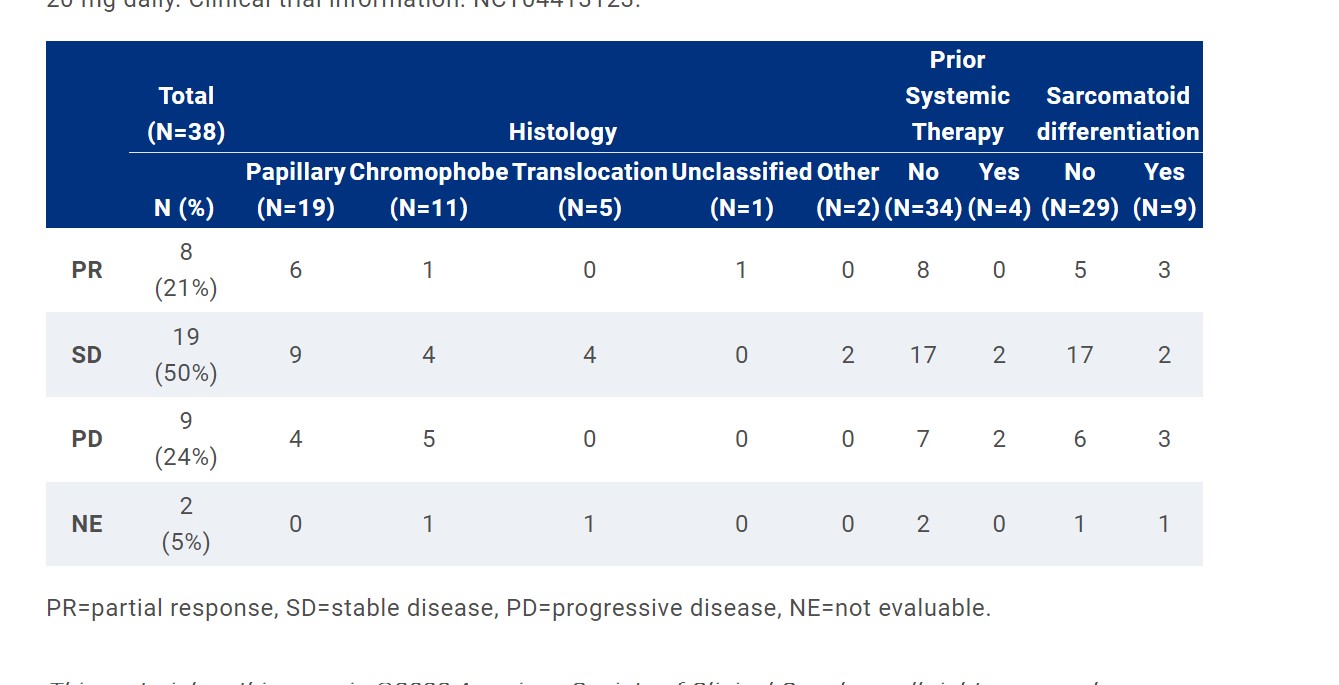
CONCLUSIONS:
The study suggests activity for this combination in patients with RCCvh particularly among those without chromophobe histology. An additional cohort of 20 pts is enrolling with Cabo starting dose of 20 mg daily. Clinical trial information: NCT04413123.
ABSTRACT 4541
-Core biopsy (bx) accuracy and safety of biopsy and preoperative immunotherapy in predicting histological subtype and nuclear grade in ECOG-ACRIN EA8143 perioperative nivolumab (nivo) versus observation in patients (pts) with renal cell carcinoma (RCC) undergoing nephrectomy.
Naomi B. Haas, Se Eun Kim, David F. McDermott, Viraj A. Master, Sabina Signoretti, Mahmut Akgul, Nick Baniak, Elsa Li Ning Tapia, Matthew Palmer, Hamid Emamekhoo, Bradley C. Leibovich, Brian M. Shuch, Anil Kapoor, M. Dror Michaelson, Gennady Bratslavsky, Michael Anthony Carducci, Mohamad E. Allaf
ORGANIZATIONS
Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, Dana Farber Cancer Institute-ECOG-ACRIN Biostatistics Center, Boston, MA, Beth Israel Deaconess Medical Center, Dana-Farber/Harvard Cancer Center, Boston, MA, Winship Cancer Institute of Emory University, Atlanta, GA, Brigham and Women’s Hospital, Dana-Farber Cancer Institute, and Harvard Medical School, Boston, MA, Albany Medical Center, Albany, NY, University of Saskatchewan, Saskatoon, SK, Canada, MD Anderson Cancer Center, Houston, TX, University of Pennsylvania, Philadelphia, PA, University of Wisconsin Carbone Cancer Center, Madison, WI, Mayo Clinic, Rochester, MN, Institute of Urologic Oncology, David Geffen School of Medicine at UCLA, Los Angeles, CA, McMaster University and Juravinski Cancer Centre, Hamilton, ON, Canada, Massachusetts General Hospital, Boston, MA, SUNY Upstate Medical University, Department of Urology, Syracuse, NY, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD, James Buchanan Brady Urological Institute, Dept. of Urology, Johns Hopkins University School of Medicine, Baltimore, MD
BACKGROUND PROSPER is a phase 3 National Clinical Trials Network study that accrued pts with clinical stage ≥T2 or TanyN+ RCC of any histology planned for radical/ partial nephrectomy. Pts were randomized to perioperative nivo followed by primary tumor resection and 9 cycles of nivo postoperatively or standard surgery followed by observation. To address concerns that core kidney bx might 1) not provide accurate diagnosis and 2) delay surgery due to adverse events (AEs) or scheduling, the study was amended from requiring all patients to undergo core bx prior to treatment for confirmation of RCC, to only require bx in patients randomized to the nivo arm. Herein, we report the accuracy and safety of primary tumor core bxs in predicting final histology and nuclear grade by both site and central pathology review as well as time from enrollment to surgery. We also report AEs of preoperative nivo.
METHODS: Concordance of both core bx and primary tumor by site and central pathology review of histology and grade (1-2 vs 3-4) are reported, along with the Cohen’s Kappa value, which measures the agreement and concordance (kappa=0 is no concordance and 1 is highest). AEs relating to core bxs and preoperative nivo, as well as time from enrollment to surgery for each arm, comparing pre- and post-amendment (dropping bx requirement in surgery alone arm) are also reported.
RESULTS: 387/404 pts in the nivo arm and 171/415 pts in the surgery alone arm had core bxs. 632 patients had both central pathology and site review available. 41 of all randomized patients (819) were considered as non-RCC and 26/41 were identified via bx. The median times from enrollment to surgery for nivo and control arms pre-amendment were 32d vs 19 d, and post-amendment were 21 d vs 14 d, respectively. The median (25th-75th percentile) number of days from last preoperative nivo to surgery was 14 d (9-20). AEs related to core bx, generally from bleeding, were reported in 13/558 (2.3%) pts. 2/13 bxs resulted in life-threatening complications. 21/353 (6%) of pts receiving nivo pre-surgery had ≥ grade 3-5 AE attributed to nivo. 181/353 (51%) pts had any grade AE attributed to nivo. Concordance between bxs and primary tumor pathologies for determining histological subtype was Kappa = 0.62. Agreement between central pathology and originating site review of primary tumor for determining nuclear grade was Kappa = 0.56, and concordance of histology was Kappa = 0.78.
CONCLUSIONS: The PROSPER trial use of core bxs in advance of neoadjuvant therapy was generally safe, largely consistent with primary tumor histology and grade, and did not delay resection of the primary tumor. AEs of preoperative nivo were consistent with nivo AEs in metastatic disease. This approach is valid for future neoadjuvant trials. Clinical trial information: NCT03055013.
ABSTRACT 4551
-
CD8 cell PET imaging with 89-Zr-crefmirlimab berdoxam (crefmirlimab) in patients with metastatic renal cell carcinoma (mRCC) receiving checkpoint inhibitors (CPIs): Association with response and tissue CD8 expression.
Sumanta Kumar Pal, Przemyslaw Twardowski, Delphine L. Chen, Evan Thomas Hall, David Hays, Ian Wilson, Kristin Schmiedehausen, Michael Ferris, William Le, Michael A. Postow
ORGANIZATIONS
City of Hope, Duarte, CA, John Wayne Cancer Institute, Santa Monica, CA, University of Washington, Seattle, WA, CARTI Cancer Center, Little Rock, AZ, ImaginAb, Inglewood, CA, Memorial Sloan Kettering Cancer Center, New York, NY
BACKGROUND Novel imaging modalities using frequently expressed RCC antigens, such as CAIX, have shown promise in early-stage disease (Shuch B et al ASCO GU 2023). In advanced RCC, no tissue-based biomarkers have been well established to predict outcome with contemporary regimens, e.g., checkpoint inhibitors (CPIs) or targeted therapy (TT). We hypothesize that functional imaging of CD8 T-cells (CD8s) with crefmirlimab (a ~80 kDA 89Zr-labelled minibody with high affinity for CD8) may predict response given the essential role of CD8T-cells (CD8s) in mediating CPI response.
METHODS: Eligible pts had pathologically verified RCC, metastatic disease and an intent to initiate standard of care CPI therapy. Patients received crefmirlimab PET/CT within 1 wk of CPI infusion and 4-6 weeks after initiating therapy. Baseline biopsy was mandated, along with repeat biopsy 0-2 weeks following the second PET/CT scan. PET signal was characterized as SUVmax, SUVpeak and SUVmean of the biopsied lesions, up to 5 index lesions and representative CD8 avid lymph nodes. Mean SUVmax in responders and non-responders were compared using students t-test (1-sided). CD8 expression in tissue was characterized as the number of positive cells per mm2; PET avidity and CD8 expression were compared using the Spearman correlation coefficient.
RESULTS: 17 pts (9 M: 8 F) were enrolled; most pts had clear cell histology (12; 71%) followed by unclassified (3; 17%) and papillary (2; 12%). The most commonly rendered CPI-based regimens were nivolumab alone (6 pts; 35%) and cabozantinib/nivolumab (3 pts; 17%). Follow-up data was available in 15 of the patients. By RECIST v1.1, 3 of 15 patients were classified as responders (best overall response [BOR] of complete response or partial response) and 12 patients were classified as non-responders (BOR of stable disease or progressive disease). Average SUVmax, SUVpeak and SUVmean per patientamong all quantified index lesions and representative lymph nodes were 10.02, 6.95 and 6.11 for baseline and 8.82, 6.23 and 5.39 during treatment, respectively. Average SUVmax at baseline was 14.68 in responders to CPI and 8.28 in non-responders (P=0.006). On treatment SUVmax was 10.93 in responders to CPI and 8.22 in non-responders (P=0.19). A strong correlation between CD8 expression in baseline tissue and normalized SUVmean was observed (r=0.77; 95%CI 0.53-0.91).
CONCLUSIONS: To our knowledge, this is the first series in RCC to demonstrate that functional imaging of immune cells (here, CD8s) may segregate response to CPIs, with responders having a higher baseline SUV and a larger decrement in SUV with therapy. Our results are bolstered by a significant correlation between tissue and imaging CD8 expression. Larger studies are underway to validate this noninvasive strategy. Clinical trial information: NCT03802123.
ABSTRACT 4554
-
89Zr-DFO-girentuximab for PET/CT imaging of clear cell renal cell carcinoma: Results from phase 3 ZIRCON study.
Brian M. Shuch, Allan J. Pantuck, Jean-Christophe Bernhard, Michael Morris, Viraj A. Master, Andrew Mark Scott, Charles Van Praet, Clément Bailly, Bulent Onal, Tamer Aksoy, Robin Merkx, David M. Schuster, Sze Ting Lee, Neeta Pandit-Taskar, Alice C. Fan, Libuse Tauchmanova, Karl Schmidt, Kavita Vadali, Colin Hayward, Peter Mulders
ORGANIZATIONS
Institute of Urologic Oncology, David Geffen School of Medicine at UCLA, Los Angeles, CA, Institute of Urologic Oncology, University of California Los Angeles, Los Angeles, CA, CHU Bordeaux, Bordeaux, France, Advanced Molecular Imaging and Therapy, Glen Burnie, MD, Emory University, Atlanta, GA, Department of Molecular Imaging and Therapy, Austin Health and University of Melbourne; Olivia Newton-John Cancer Research Institute and La Trobe University, Heidelberg, Australia, Department of Urology, Universitair Ziekenhuis, Gent, Belgium, CHU de Nantes, Hotel Dieu - HME, Nantes, France, Istanbul University- Cerrahpasa, Cerrahpasa Medical Faculty, Urology Department, Istanbul, Turkey, Istanbul Training and Research Hospital, Istanbul, Turkey, Department of Medical Imaging, Radboud University Medical Center, Nijmegen, Netherlands, Department of Radiology and Imaging Sciences, Division of Nuclear Medicine and Molecular Imaging, Emory University, Atlanta, GA, Department of Molecular Imaging and Therapy, Austin Health and University of Melbourne; Olivia Newton-John Cancer Research Institute and La Trobe University, Melbourne, Australia, Memorial Sloan Kettering Cancer Center, New York, NY, Cancer Center, Stanford, CA, Telix Pharmaceuticals, North Melbourne, Australia, ABX-CRO Advanced Pharmaceutical Services Forschungsgesellschaft mBH, Dresden, Germany, Radboud University Medical Center, Nijmegen, Netherlands
BACKGROUND Conventional tools (eg, CT, MRI, biopsy) have limitations for characterizing renal mass histology; approx. 25% of patients with an indeterminant renal masses (IDRM) << 4cm undergo surgery for benign disease. Accurate noninvasive techniques to risk stratify the IDRM remains an unmet need. Girentuximab is a monoclonal antibody that targets carbonic anhydrase IX (CAIX), an enzyme highly expressed in clear cell renal carcinoma (ccRCC). Radiolabeled 89Zr-DFO-girentuximab is highly specific for CAIX and can aid differentiation between ccRCCs and other renal lesions. ZIRCON evaluated the performance of 89Zr-DFO-girentuximab PET/CT for detection of ccRCC.
METHODS: In this open label, multicenter trial, patients with an IDRM (≤7cm; cT1) who were scheduled for partial nephrectomy within 90 days from planned 89Zr-DFO-girentuximab administration were eligible. Enrolled patients received a single dose IV (37 MBq±10%; 10mg girentuximab) on Day 0 and underwent PET/CT imaging on Day 5 (±2d). Blinded central histology review determined ccRCC status. The co-primary objectives were to evaluate both the sensitivity and specificity of 89Zr-DFO-girentuximab PET/CT imaging in detecting ccRCC in patients with IDRM, using histology as the standard of truth. Key secondary objectives included sensitivity and specificity of TLX250-CDx PET/CT imaging in the subgroup of patients with IDRM ≤4cm (cT1a). Other secondary objectives included positive and negative predictive values, and evaluation of safety and tolerability. The Wilson 95% confidence intervals (CI) lower bound for sensitivity and specificity had to be >70% and 68% respectively for ≥2 independent readers to declare the study successful.
RESULTS: 300 patients received 89Zr-DFO-girentuximab (mean age, 62±12y; 71% Male). Of 288 patients with central histopathology of surgical samples, 193 (67%) had ccRCC, and 179 (62%) had cT1a. Of 284 evaluable patients, the average across all 3 readers for sensitivity and specificity was 86% [80%, 90%] and 87% [79%, 92%] resp. for coprimary, and 85% [77%, 91%] and 90% [79%, 95%] resp. for key secondary endpoints. For all evaluable patients, positive and negative predictive values were ≥ 91.7% and ≥ 73.7%, resp. PET+ ccRCC had higher mean CAIX expression compared with PET- ccRCC patients (p << 0.05). Sensitivity and specificity were consistent with masses ≤2cm (n=46) of which, 26 were ccRCC+, 13 ccRCC−, and 3 unevaluable at central histopathology. Of 263 adverse events (AEs) in 124 patients, 2 AEs of mild intensity were treatment related.
CONCLUSIONS: ZIRCON study confirms 89Zr-DFO-girentuximab PET/CT is a well-tolerated and accurate modality for noninvasive identification of ccRCC in IDRM. This tool could be included in the diagnosis/management of patients with IDRM, limiting unnecessary treatment of benign lesions. Clinical trial information: NCT03849118.
ABSTRACT 4560
-
Patient priorities and expectations of systemic therapy in metastatic renal cell carcinoma.
Dena Battle, Ulka N. Vaishampayan, Pavlos Msaouel, Sumanta Kumar Pal, Tian Zhang, Michael D. Staehler
ORGANIZATIONS
KCCure, Alexandria, VA, Division of Hematology/Oncology, University of Michigan, Ann Arbor, MI, University of Texas MD Anderson Cancer Center, Houston, TX, City of Hope, Duarte, CA, University of Texas Southwestern Medical Center, Dallas, TX, University of Munich, Muenchen, Germany
BACKGROUND As treatment options for metastatic Renal Cell Carcinoma (RCC) have increased in number, selecting therapy has become more complicated. When prioritizing agents today, guidelines recommend selection based primarily on risk classification, as well as consideration of efficacy data, patient characteristics, quality of life, cost, and patient preference. Understanding how patients prioritize treatment selection and define treatment success is crucial to improving patient/provider communication and to improving future drug development.
METHODS: The survey was developed by the Kidney Cancer Research Alliance (KCCure) and was broadcast between 07/2022 and 09/2022 to patients via website, mailing lists and social media platforms. Those who agreed to participate were surveyed for demographics (age, gender, race, income, country) and clinical characteristics (date of the diagnosis, disease stage, treatment history). Descriptive statistics summarized the survey data.
RESULTS: 399 out of 1,062 patients surveyed had metastatic disease. 80% of patients were receiving or had received systemic therapy, 20% of patients had not yet received systemic therapy. 52% were female and 48% were male, with a median age of 57 years (range 28-86). Patients identified as white (89%) and living in the United States (86%). 69% of patients reported that they did not know their IMDC or risk status, 10% were favorable risk, 11% were intermediate risk and 10% were poor risk. When asked to select the most important outcome for treatment selection on a rank-choice scale from 1 to 8, the chance to eliminate all evidence of disease (complete response) scored highest (6.6), followed by durability of response (5.1), improved quality of life (5.0), rapid reduction of tumors (4.9), ability to go off therapy (4.2), low risk of toxicity (4.0) and reduction of tumor symptoms (4.0). Patients ranked low cost as the least important factor in selecting treatment (2.3). 70% of patients defined “long-term" response to therapy as five years or longer, and over a quarter of patients (26%) defined long-term response as 10 years or longer. When asked to define treatment success, patients rank radiological reduction in tumor size (83%) as the most important factor, followed by stable disease (67%), improved quality of life (48%) and the ability to return back to work (22%). The lowest ranked choice was “I just trust my doctor” (17%).
CONCLUSIONS: Most patients are not familiar with their risk classification and may not realize the significance of this factor in treatment selection. Patients rank complete response as the most important outcome/desire when considering treatment options. Cost is the least important factor for patients in selecting treatment. Patient perceptions of long-term response to therapy may differ from provider perceptions. More research is needed to improve patient/provider communication in the therapy selection process.