Impact of Mycobacterial Infections on Outcomes of Patients with Metastatic Renal Cell Carcinoma: A Case Series
1 Division of Medical Oncology, University of Colorado Anschutz Medical Campus, Aurora, CO, USAABSTRACT
Objective: Renal cell carcinoma (RCC) is a common cancer, and mycobacterial infections (MBI) are some of the most prevalent infections in the world. Little is known about their overlap and how MBI might affect outcomes of RCC. The objective of this series was to explore the relationship between MBI and RCC in terms of patient survival and treatment response.
Methods: Institutional records were searched for patients with diagnoses of kidney cancer and MBI. Patients with histologically confirmed metastatic RCC diagnosed up to the date of IRB approval were included. Patient demographics, tumor characteristics, clinical data, and treatment modalities and durations were collected and analyzed. Primary outcome was overall survival.
Results: 5 patients were included. Median overall survival (mOS) of patients with RCC and subsequent diagnosis of MBI was ≥62 months. mOS of patients carrying both diagnoses without concern for temporal relation was ≥24 months.
Conclusion: Development of an MBI during RCC malignancy treatment may positively impact on therapy response and improve OS. Improved TKI response duration may be related to upregulation of VEGF and angiogenesis seen as a downstream consequence of the immune response to mycobacterial infection.
INTRODUCTION
Renal cell carcinoma (RCC) is among the top 10 cancers for both men and women, with nearly 74,000 new diagnoses expected in the US this year¹. Even with recent advances, the 5year relative survival for metastatic RCC remains low, at approximately 12%¹. Patients with RCC and other malignancies are at higher risk for infection development, but the impact of viral and bacterial infections on outcomes of malignancy remains controversial.
Physicians and researchers have observed the impact of spontaneous infections on cancers for over 150 years. In 1867, Busch in Germany reported on a cancer that went into remission after a bout of erysipelas. At the turn of the 20th century, this knowledge was harnessed by Dr. William Coley, often referred to as the “father of immunotherapy of cancer.” Coley inoculated patients with Streptococcus pyogenes and Serratia marcescen bacteria and found patients with sarcomas could often be put into deep and durable remissions². More recent data on the impact of infections are conflicting. Within retrospective studies of post-operative infections in glioblastoma multiforme, De Bonis et al found that post-operative infection led to a significant survival advantage³, whereas Bohman et al found that it did not⁴. Furthermore, while studies have shown that postoperative intra-abdominal infections in patients with stage II colon cancer have a negative impact on disease-free survival and disease-specific survival⁵ and surgical site infections following resection of T4N0-2M0 colon cancers are associated with an increased risk of intraabdominal recurrence and worse survival⁶, post-orthotopic liver transplantation infections tend to improve the outcome of hepatocellular carcinoma patients⁷ and postoperative empyema seems to improve survival in lung cancer⁸.
Mycobacterium tuberculosis is one of the most common infections worldwide, affecting about one quarter of the world’s population⁹. Non-tuberculous mycobacterial infections (MBI) are also common10-14. In our institution, we observed that patients with metastatic RCC (mRCC) who at some point in their treatment developed mycobacterial pulmonary infections appeared to have prolonged overall survival (OS) when compared to what is expected in the general mRCC population. This has not been previously reported in the literature. Our objective was to assess the correlation between the presence of prior or concurrent MBI and patient survival and treatment response in mRCC.
METHODS
After local IRB approval, institutional electronic medical records and databases were queried for patients with a diagnosis of both “malignant neoplasm of kidney, except renal pelvis” (ICD-10 C.64) and either “infection due to other mycobacteria” (ICD-10 A.31) or “respiratory tuberculosis” (ICD-10 A.15). Patient records were then assessed for accuracy of aforementioned diagnostic criteria and for presence of metastatic RCC. Patients with histologically confirmed mRCC with clear cell or non-clear cell histology were included; patients without metastatic disease were excluded. Patients who were diagnosed with RCC up to the date of local IRB approval were included.
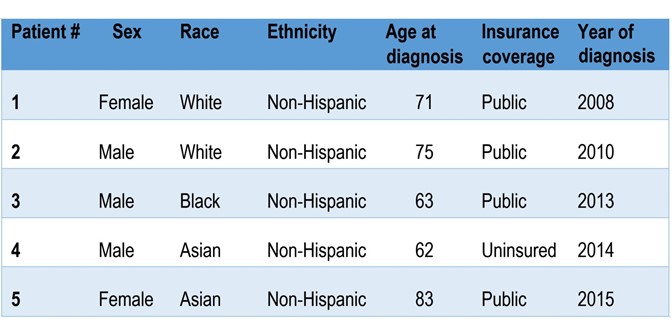 Table 1 | Baseline patient characteristics.
Table 1 | Baseline patient characteristics.
The following data were collected: baseline patient demographics, baseline tumor characteristics, clinical data, treatment data, time of diagnosis of MBI in relation to diagnosis of RCC, treatment for MBI, outcomes including duration of response to anticancer therapy before and after MBI infection, and OS, defined as the time from diagnosis of mRCC to the time of death from RCC. Results Twenty-seven patients were initially identified throughout the University of Colorado Health system applying the above diagnostic criteria. Sixteen were excluded due to only nonmetastatic localized disease, five did not truly carry a diagnosis of renal cell carcinoma, and one was excluded for squamous cell differentiation and a lack of clarity surrounding the primary tumor of origin. Five patients were therefore assessed in this series, with baseline patient characteristics as described in Table 1. Two female and three male patients, ages 62- 83, were included in the series.
Characteristics of the patients’ mRCC are outlined in Table 2. One patient harbored sarcomatoid features. Patients initially presented at both localized and metastatic disease stages. Three patients had International Metastatic RCC Database Consortium (IMDC) and Memorial Sloan-Kettering Cancer Center (MSKCC) Intermediate Risk category disease, one had poor risk disease, and one had favorable risk disease. Sites of metastases included lymph nodes, bone, lung, liver, soft tissue, brain, and in the nephrectomy bed.
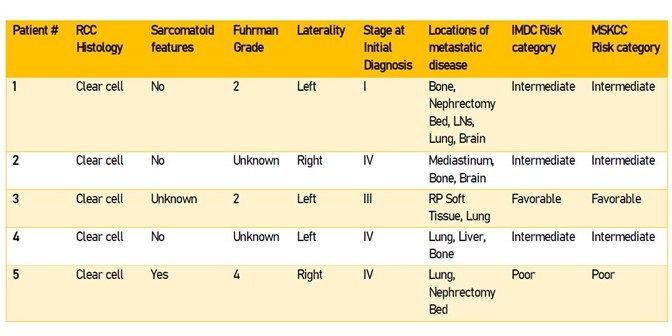
TABLE 2 | Renal cell carcinoma characteristics. Key: IMDC – International Metastatic RCC Database Consortium (1 point each for <1 year from diagnosis to start of therapy, Karnofsky Performance Status <80%, Hemoglobin < lower limit of normal (LLN), calcium > upper limit of normal (ULN), neutrophils > ULN, platelets > ULN; 0 points favorable risk, 1-2 points intermediate risk, 3+ points poor risk). MSKCC – Memorial Sloan-Kettering Cancer Center (1 point each for <1 year from diagnosis to start of therapy, Karnofsky Performance Status <80%, Hemoglobin < LLN, calcium > 10 mg/dL, LDH > 1.5x ULN; 0 points favorable risk, 1-2 points intermediate risk, 3+ points poor risk.
The treatment modalities used are included below (Table 3). Four patients had prior nephrectomy. Two patients received local radiation therapy to metastatic sites. Four patients received systemic therapy, including tyrosine kinase inhibitors (TKIs) (pazopanib, sorafenib, axitinib, cabozantinib), immune checkpoint inhibitors (nivolumab), and experimental drugs. Two patients enjoyed durations of 32 and 39 months of systemic therapy with TKIs prior to their MBI diagnosis. Four patients received systemic therapies subsequent to their infection diagnosis, with durations ranging from 24 months to 72 months of therapy. One patient previously had pulmonary tuberculosis, two patients were diagnosed with an MBI at the same time as their mRCC diagnosis, and two patients were diagnosed after their mRCC diagnosis. Only one patient underwent treatment of MBI diagnosed after RCC diagnosis: standard triple therapy of ethambutol, isoniazid, and rifampin. Overall survival ranged from 2 to 117 months.
DISCUSSION
Mycobacterial infections are common infections clinically ranging from asymptomatic to morbid. Generally speaking, infections have negative impact on patients in the short term, but it is unknown whether there is a positive impact for patients with mRCC. While the cohort of patients in this retrospective analysis was small, patients who developed MBI after mRCC diagnosis seemed to have prolonged PFS and OS.
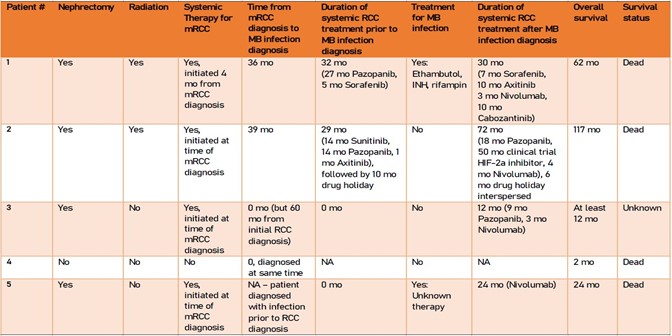 Table 3 | Treatment of mRCC and MBI data. Key: INH – isoniazid, TB – tuberculosis, LTBI – latent tuberculosis infection, mo –
months, MB – mycobacterial, NA – not applicable.
Table 3 | Treatment of mRCC and MBI data. Key: INH – isoniazid, TB – tuberculosis, LTBI – latent tuberculosis infection, mo –
months, MB – mycobacterial, NA – not applicable.
Within the past decade, there have been vast improvements in mRCC treatment options that prolong life and improve quality of life. The median overall survival (mOS) for advanced and metastatic RCC is reported to be approximately 20 – 30 months (15-19). Analysis of our cohort found that, in the three patients who were diagnosed with MBI after already diagnosed with RCC, their OS ranged from 12 to 117 months. The mean and median OS in among these patients were 63.7 months and 62 months, respectively. If we also include the two patients who either had a history of MBI or were diagnosed with the infection at the same time as diagnosis of the RCC, the mean OS and median OS decreases to 43.4 months and 24 months, respectively. In summary, if a patient with mRCC was diagnosed concurrently or prior to MBI, OS was not necessarily improved. However, patients in our cohort who developed MBI after diagnosis of RCC appeared to have substantially improved OS when compared with that of the general population of patients with mRCC and demonstrated longer duration of treatment and disease stability following MBI.
The beneficial results seen in patients who were diagnosed with MBI after diagnosis of RCC may be confounded by other factors. Two patients had long treatment responses to TKIs prior to MBI, which reflects favorable biology of RCC; however, it is noteworthy that both these patients also had brain metastases, which normally would confer poor prognosis. Patient 5 harbored sarcomatoid features and had poor risk disease, yet she an OS of 24 months, much longer than what would be expected for sarcomatoid RCC.
We had predicted that patients with MBI would respond better to immune checkpoint inhibitors based on their increased immune system activation, but interestingly patients in this cohort received PD-1 checkpoint inhibitor therapy for only a short time prior to progressing. Patients 1 and 2 had the most impressive OS, at 62 and 117 months, and had prolonged responses to VEGFR TKI therapies both prior to and after MBI. Additionally, these patients maintained excellent control of their mRCC in the chest, but had recurrences in the brain, requiring local treatments. It is interesting to consider if the infection perhaps conferred some benefit systemically that is unable to cross the blood brain barrier into the central nervous system (CNS) to then affect the same benefit.
There are no existing models to demonstrate the mechanism of this possible benefit. The pathogenesis of pulmonary disease due to M. tuberculosis is well understood, and the pathogenesis of other MBI are presumed to have similarities to that of tuberculosis (20). The cellular response first involves alveolar macrophages, where the mycobacterium is taken up and proliferates within their vacuoles as an intracellular pathogen via immune-evasion mechanisms. These macrophages activate T lymphocytes and NK cells via cytokine release, and the mycobacterial antigens are also presented on macrophages to T lymphocytes leading to subsequent expansion of T lymphocyte clones. IL-2, IL-12, TNF-alpha, and IFN-gamma are major players in the immune response to MAC, and IL-6, IL-10, and TGF-beta are important in modulating the immune response (21). TGF-beta, a regulator of the immune response, is also involved in upregulation of VEGF and angiogenesis (22, 23). During MBI, not only is TGF-beta released locally for regulation and suppression of the immune system in MBI, but there are higher levels of TGF-beta found systemically in the blood of patients with MBI (24, 25). Theoretically, if TGF-beta is elevated systemically to mitigate the proinflammatory cytokines released in response to the infection, then VEGF and resultant angiogenesis should also be upregulated throughout the body and throughout sites of metastatic disease. This upregulation could possibly then make the tumor cells of the RCC more susceptible to our standard anti-angiogenic therapies with TKIs which inhibit VEGF and TGF-beta pathways (26)). In support of this hypothesis, Patients 1 and 2 had prolonged responses (27 and 18 months, respectively) to TKI therapy following their diagnoses of MBI, even when the TKI was used as third- and fourth-line therapies and beyond, when we might expect time to progression on these drugs to be quite low. Another hypothetical mechanism that could explain a local protective response would be related to the local damage and relative hypoxia induced by a mycobacterial pulmonary infection. Hypoxia should locally upregulate hypoxia-inducible transcription factors (HIFs), which are integral in the subsequent upregulation of VEGF-A and angiogenesis (27-29). Again, this may then allow the RCC to be more susceptible to TKIs, as seen in Patients 1 and 2, and could also explain Patient 2’s remarkable duration of response of 50 months to the HIF-2a inhibitor he received as part of a phase I clinical trial. The role of TGF-beta, HIF, VEGF, and angiogenesis, and the response to therapies directed against angiogenesis could also help to explain why the patients with history of MBI prior to their RCC diagnosis did not derive the same benefit from TKI therapy as the patients who already had RCC at time of infection diagnosis.
This series shows a trend toward improved outcomes in patients who experienced an MBI during the time of treatment for mRCC, although we cannot draw generalizable conclusions due to the small cohort of patients. By expanding our search to multiple institutions, one would expect a broader distribution of patients with both favorable and unfavorable tumor characteristics and clinical characteristics, and a wider range of patients who experienced their MBI before, simultaneously, and after mRCC diagnosis. It would be interesting to assess whether a benefit of MBI is seen across all risk stratifications, different histologies, treatment modalities, etc. Additional lines of query could then include whether other types of infections have impact on outcomes in mRCC, or whether a protective benefit against development of metastases could be seen in the brain if the MBI was serious enough to involve the CNS (as in the case of tuberculous meningitis or tuberculoma).
CONCLUSION
Renal cell carcinoma is a common cancer, and tuberculous and non-tuberculous MBI are among the most common infections throughout the world; the intersection of these two diagnoses brought into question the impact of the latter on the former. This series of five patients within a single institution revealed that simply carrying a diagnosis of both mRCC and MBI did not improve OS, but suggested that the development of MBI during ongoing malignancy had an impact on response to TKI therapy and improved OS. Additional consideration of these findings within a larger cohort of patients is necessary, as it may offer further insight into the issue, even assisting in prognostication of disease outcomes for individuals or prediction of their responsiveness to specific therapies.
KEYWORDS: Kidney Cancer • MAC • Mycobacterial Infection • Renal Cell Carcinoma • Tuberculosis •
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
- 2. Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res. 1991(262):3-11.
- 3. De Bonis P, Albanese A, Lofrese G, de Waure C, Mangiola A, Pettorini BL, et al. Postoperative infection may influence survival in patients with glioblastoma: simply a myth? Neurosurgery. 2011;69(4):864-8; discussion 8-9.
- 4. Bohman LE, Gallardo J, Hankinson TC, Waziri AE, Mandigo CE, McKhann GM, 2nd, et al. The survival impact of postoperative infection in patients with glioblastoma multiforme. Neurosurgery. 2009;64(5):828-34; discussion 34-5.
- 5. Sánchez-Velázquez P, Pera M, Jiménez-Toscano M, Mayol X, Rogés X, Lorente L, et al. Postoperative intra-abdominal infection is an independent prognostic factor of disease-free survival and disease-specific survival in patients with stage II colon cancer. Clin Transl Oncol. 2018;20(10):1321-8.
- 6. Klaver CEL, Wasmann K, Verstegen M, van der Bilt JDW, Nagtegaal ID, van Ramshorst B, et al. Postoperative abdominal infections after resection of T4 colon cancer increase the risk of intra-abdominal recurrence. Eur J Surg Oncol. 2018;44(12):1880-8.
- 7. Chao JS, Zhao SL, Ou-Yang SW, Qian YB, Liu AQ, Tang HM, et al. Post-transplant infection improves outcome of hepatocellular carcinoma patients after orthotopic liver transplantation. World J Gastroenterol. 2019;25(37):5630-40.
- 8. Ruckdeschel JC, Codish SD, Stranahan A, McKneally MF. Postoperative empyema improves survival in lung cancer. Documentation and analysis of a natural experiment. N Engl J Med. 1972;287(20):1013-7.
- 9. Global tuberculosis report 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO.
- 10. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881-6.
- 11. Brode SK, Daley CL, Marras TK. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: a systematic review. Int J Tuberc Lung Dis. 2014;18(11):1370-7.
- 12. Henkle E, Hedberg K, Schafer S, Novosad S, Winthrop KL. Population-based Incidence of Pulmonary Nontuberculous Mycobacterial Disease in Oregon 2007 to 2012. Ann Am Thorac Soc. 2015;12(5):642-7.
- 13. Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182(7):970-6.
- 14. Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182(7):977-82.
- 15. George DJ, Hessel C, Halabi S, Michaelson MD, Hahn O, Walsh M, et al. Cabozantinib Versus Sunitinib for Untreated Patients with Advanced Renal Cell Carcinoma of Intermediate or Poor Risk: Subgroup Analysis of the Alliance A031203 CABOSUN trial. Oncologist. 2019;24(11):1497-501.
- 16. Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722-31.
- 17. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115-24.
- 18. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277-90.
- 19. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380(12):1116-27.
- 20. Good RC, Snider DE, Jr. Isolation of nontuberculous mycobacteria in the United States, 1980. J Infect Dis. 1982;146(6):829-33.
- 21. Domingo-Gonzalez R, Prince O, Cooper A, Khader SA. Cytokines and Chemokines in Mycobacterium tuberculosis Infection. Microbiol Spectr. 2016;4(5).
- 22. Benckert C, Jonas S, Cramer T, Von Marschall Z, Schäfer G, Peters M, et al. Transforming growth factor beta 1 stimulates vascular endothelial growth factor gene transcription in human cholangiocellular carcinoma cells. Cancer Res. 2003;63(5):1083-92.
- 23. Wahl SM, Allen JB, Wong HL, Dougherty SF, Ellingsworth LR. Antagonistic and agonistic effects of transforming growth factor-beta and IL-1 in rheumatoid synovium. J Immunol. 1990;145(8):2514-9.
- 24. Ovrutsky AR, Merkel PA, Schonteich E, Bai X, Kinney W, Iseman MD, et al. Patients with non-tuberculous mycobacterial lung disease have elevated transforming growth factor-beta following ex vivo stimulation of blood with live Mycobacterium intracellulare. Scand J Infect Dis. 2013;45(9):711-4.
- 25. Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner JJ. Enhanced production of TGF-beta by blood monocytes from patients with active tuberculosis and presence of TGF-beta in tuberculous granulomatous lung lesions. J Immunol. 1995;154(1):465-73.
- 26. Yamaoka T, Kusumoto S, Ando K, Ohba M, Ohmori T. Receptor Tyrosine Kinase-Targeted Cancer Therapy. Int J Mol Sci. 2018;19(11).
- 27. Kuwai T, Kitadai Y, Tanaka S, Onogawa S, Matsutani N, Kaio E, et al. Expression of hypoxia-inducible factor-1alpha is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer. 2003;105(2):176-81.
- 28. Maxwell PH, Pugh CW, Ratcliffe PJ. Activation of the HIF pathway in cancer. Curr Opin Genet Dev. 2001;11(3):293-9.
- 29. Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677-84.
Declarations and Conflict of Interest:
CORRESPONDENCE
Rebecca C Shay, DO – First author, corresponding author, Hematology/Oncology Fellow