Dissecting the role of lymphadenectomy in the management of renal cell carcinoma:past, present, and future
ABSTRACT
Lymph node involvement in renal cell carcinoma (RCC) portends a poor prognosis. However, the role of lymph node dissection (LND) at the time of tumor resection is not fully understood. Conflicting data have been published regarding the survival implications of LND during RCC surgery, and the optimal patient population for which LND might be beneficial has yet to be identified. Based on recent data characterizing the outcomes of node-positive RCC, some have advocated for revising the current staging guidelines to better reflect these findings. Given the paucity of high-quality evidence supporting or refuting the routine use of LND in RCC, further research is needed to shed light on this important topic. There are a number of ongoing clinical trials evaluating the role of perioperative (neoadjuvant and adjuvant) systemic therapy, which include patients with node-positive RCC, and will serve to guide changes in treatment practices for this patient population moving forward.
INTRODUCTION
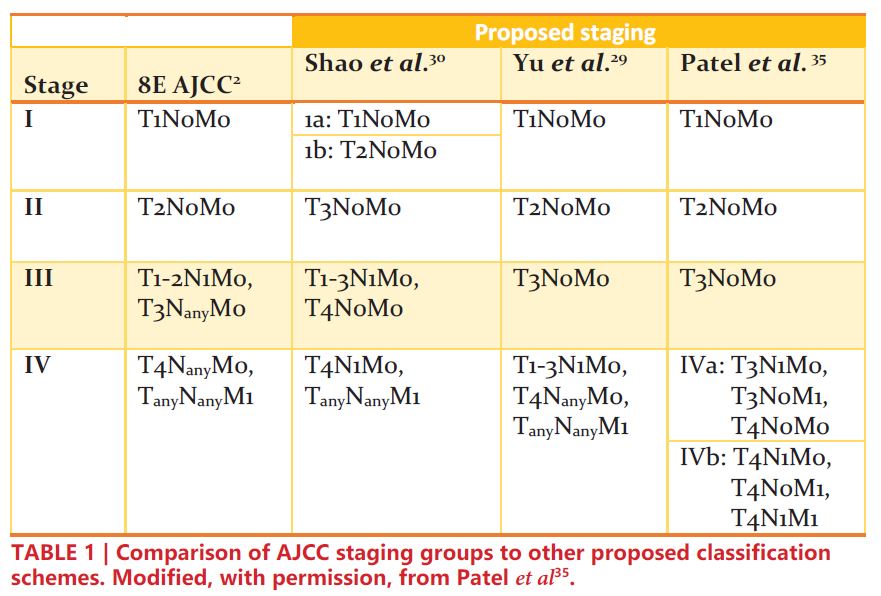
Renal cell carcinoma (RCC) is one of the most common solid organ malignancies, with over 74,000 new cases and 15,000 deaths anticipated in the United States in 2020 alone1. Staging of RCC allows clinicians to characterize disease based on similar survival outcomes, which further aids in prognostication, selection of optimal treatment modalities, and clinical trial eligibility. The criteria for RCC staging as outlined by the American Joint Committee on Cancer (AJCC) are highlighted in Table 12.
For many urologic malignancies, concurrent lymph node dissection (LND) at the time of primary tumor resection offers essential treatment and diagnostic value. Removing malignant lymph nodes may significantly reduce a patient’s overall tumor burden. Furthermore, detection of positive lymph nodes critically informs the probability of disease risk stratification and the requirement for additional treatments. For example, immediate administration of androgen deprivation therapy for node-positive prostate cancer, which was detected by pelvic LND, has demonstrated clinically significant survival benefits3. Patients with pathologically node-positive bladder cancer after pelvic LND achieve a greater survival benefit from adjuvant chemotherapy compared to node-negative patients4-6. Similarly, adjuvant chemotherapy has been shown to lower recurrence rates among nonseminomatous germ cell testis cancer patients who have positive nodal disease after retroperitoneal LND7.
However, existing literature remains unclear regarding the clinical utility of LND for RCC. The American Urologic Association (AUA) and the National Comprehensive Cancer Network (NCCN) have published contemporary guidelines, but these recommendations are not supported by strong evidence. The AUA states that LND should be performed only when there is suspicion of lymphadenopathy, as LND could potentially aid in staging8, and NCCN guidelines recommend that LND should only be performed when there are palpable or enlarged lymph nodes on preoperative imaging tests9. Still, the supporting literature has not identified which patients derive the greatest benefit, if any, from LND. This uncertainty is exacerbated by unpredictable lymphatic drainage patterns of the kidneys, as well as the fact that there is no universal template for LND during kidney cancer surgery10. 11. While retrospective studies have shown a survival benefit of LND for RCC12, 13, the only randomized clinical trial to have studied LND for RCC, EORTC 30881, showed no oncologic benefit of LND with regards to overall survival, time to progression, or progression-free survival14. In this review, we consider lymph node positivity in RCC as it relates to staging, outcomes, patient selection for lymph node dissection, and the role of systemic therapy.
Patient Selection for Lymph Node Dissection
Given the uncertainty behind the benefit of LND in treating RCC, fewer urologists have been performing LND over the past decade15, 16. In an analysis of 37,279 patients who underwent radical nephrectomy for RCC between 1988 and 2015 selected from the Surveillance, Epidemiology, and End Results (SEER) registry, Kates et al. identified a 63% reduction in LND rates among localized tumors16. In 2005, LND rates in the US had fallen below 5% for all RCC surgeries17. This decrease in LND rate can, at least partially, be attributed to the clinical stage migration toward early-stage RCC (i.e. patients who are unlikely to receive LND), which has followed advancements in imaging capabilities since the 1980s18.
In a retrospective analysis of 110,963 patients with non-metastatic RCC from the National Cancer Database (NCDB), Radadia et al. reported that only 11,867 (11%) had LND at time of surgery19. Those patients undergoing LND were more likely to have clinically node-positive disease (OR: 18.68, 95% CI: 16.62 – 21.00, p<0.01) and less likely to undergo minimally invasive / robotic surgery (OR: 0.73, 95% CI: 0.64 – 0.77, p<0.01)19. In this same cohort, however, only 14.8% of patients receiving LND had clinically nodepositive disease, suggesting a large majority of patients who received LND had no preoperative evidence of nodal disease19. In a subsequent analysis of this patient population, Farber et al. showed that a disproportionate amount of LNDs were performed for low-stage RCC. Surgeons performed LND in 5% and 23% of patients with pT1 and pT2 RCC, respectively, despite lymph node involvement in only 1.1% and 2.3% of cases, respectively20. This apparent overutilization of LND for lower risk renal tumors likely reflects the ambiguity surrounding guidelines and the lack of strong contemporary evidence for LND implementation.
Part of this ambiguity may reflect limitations in preoperative staging. Determining candidacy for LND currently relies heavily on clinical lymph node (cLN) status and lymph node size, as determined by preoperative imaging19, 21. Preoperative computed tomography (CT) and magnetic resonance imaging (MRI) are the primary methods used to detect nodal metastases, but have sensitivities of only 77% and 73%, respectively, and have limited ability to identify nodal micro-metastases21. Unpredictable lymphatic drainage of the kidney makes it difficult to identify a consistent template for LND, which may contribute to overlooked nodal disease on preoperative imaging22. Additionally, the correlation between cLN status and pathological lymph node (pLN) status can be difficult to determine. In a retrospective analysis of 2,954 patients with RCC who underwent either partial or radical nephrectomy with LND, only 29% of patients with lymphadenopathy on preoperative CT were confirmed to be pLN positive after LND23.
Furthermore, in EORTC 30881, only 20% of patients with palpable lymphadenopathy had nodal disease after LND14. Aside from lymph node size, some have proposed using other imaging findings to determine candidacy for LND, such as evidence of perinephric or renal sinus fat invasion on CT24. Others have proposed utilizing alternative imaging techniques to better identify LND candidates. A pilot study investigating lymphotrophic nanoparticle enhanced MRI (LNMRI) showed promising results in diagnosing pLN status, with 100% sensitivity and 96% specificity25. Clearly, current modalities for staging RCC are insufficient for determining cLN and pLN status, and more accurate and reproducible preoperative methods are needed to identify optimal LND candidates.
Outcomes in Node-Positive Disease and Implication for Staging
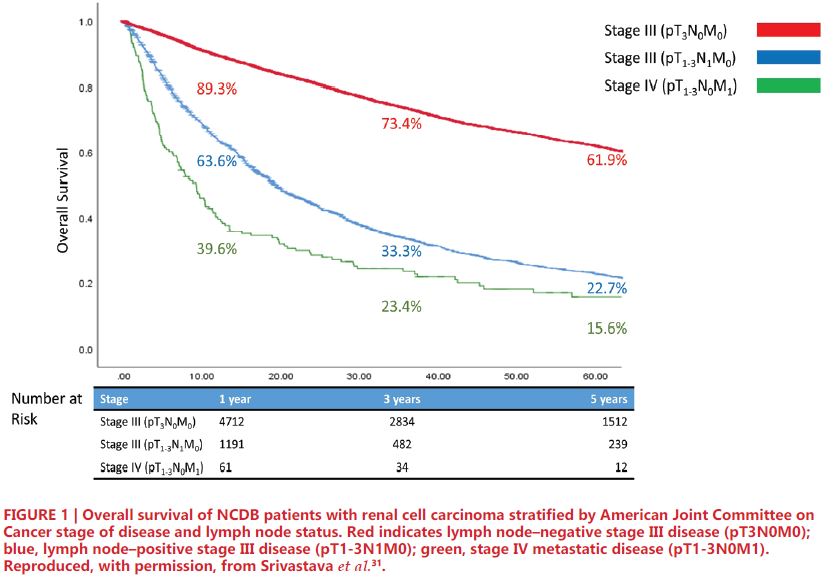
Prior studies have established that lymph node-positive disease portends worse survival in RCC26. Cancer-specific survival (CSS) in patients with lymph node positivity ranges from 21-38% at 5 years and 11-29% at 10 years, and those patients with positive nodes have nearly 8-fold higher risk of mortality compared to those with negative nodes27, 28. Notably, current AJCC staging criteria consider both pT3N0M0 (node negative) and pT1-3N1M0 (node positive) patients to have stage III RCC. However, this common grouping has been studied more closely in recent years. Several studies have proposed modification of current AJCC staging groups, lending support to the role of LND among patients with advanced RCC29-31. More specifically, these studies note that patients with node-positive Stage III RCC have survival more closely resembling Stage IV patients, including those with metastasis, rather than their node-negative Stage III counterparts. In an institutional retrospective analysis of 4,652 patients with advanced RCC, Yu et al. reported comparable oncologic outcomes between pT1-3N1M0 (Median CSS = 2.8 years, 95%CI: 1.8- 4.8 years) and pT1-3N0/xM1 (Median CSS = 2.4 years, 95%CI: 2.1-3.0 years); however, both had distinctly inferior outcomes compared to pT1-3N0M0 patients (Median CSS = not reached, 95% 10.2 – no estimate years)29. Shao et al. conducted an analogous study of 2,120 patients from a single institution which was then validated with over 74,000 patients from the SEER database. The authors noted longer overall survival (OS) for T3N0M0 compared to T1-3N1M0 (72.7% vs 38.1%), similar survival for T1- 3N1M0 and T4N0M0 (38.1% vs 36.2%), and greater survival for T1-3N1M0 compared to TanyNanyM1 disease (38.1% vs 12.6%)30. Using the NCDB, Srivastava et al. conducted a retrospective analysis of 8,988 patients with stage III/IV RCC, which compared patients with pT- 3N0M0, pT1-3N1M0, and pT1-3N0M1 disease. The results, depicted in Figure 1, showed greater 5-year OS among patients with pT3N0M0 (61.9%) compared to pT1-3N1M0 (22.7%), and similar OS between pT1-3N1M0 and pT1-3N0M1 (15.6%) disease. Of note, the results of this study also showed node positivity to be predictive of OS among Stage IIIIV patients31. Similar survival outcomes of pN1 and metastatic RCC suggest that many patients with lymph node involvement may have occult metastases at time of surgery. In a series described by Gershman et al., metastasis-free survival at 1-year was only 37%, and CSS rates were expectedly poor32.
Based on the results of these studies, some have advocated for reclassifying T1-3N1M0 RCC as stage IV instead of stage III33-35. These proposed staging revisions are shown in TABLE 1. In an era where the precise genomic and epigenetic factors are not entirely understood, cancer staging offers clinical insight into tumor biology based on objective factors. As such, in addition to its prognostic implications, revamping the classification of localized node-positive RCC could potentially better inform treatment modalities and refine eligibility for clinical trials.
Given the mortality associated with nodal disease, one might expect that LND at the time of nephrectomy would offer a survival benefit, however, mixed results have been published on this matter over the past several decades. Early work from Herrlinger et al. showed an OS advantage among patients undergoing complete LND compared to those undergoing partial or absent node dissection12, 36. Similarly, other studies found increased OS and CSS among patients with node-positive disease who underwent LND compared to those who did not13, 37, 38. To date, only one prospective, randomized phase 3 trial has assessed the utility of LND in RCC. The EORTC 30881 trial, published in 2009, showed no survival benefit among patients who underwent nephrectomy with LND compared to patients who underwent nephrectomy alone14. However, multiple limitations to this study make it difficult to interpret and implement the findings of this study for clinical practice. Most notably nearly 70% of the study population had pT1 or pT2 disease, and only 4% of patients in the trial population had nodal metastasis39. Therefore, the majority of patients in the trial were unlikely to benefit from node dissection40. EORTC 30881 was also limited in that there was no universal LND template required, and therefore results could have varied significantly based on surgeon, template, and center. Despite these shortcomings, subsequent retrospective studies attempting to clarify the impact of LND have shown similar results to EORTC 3088120, 41-44. In a study of the NCDB, Farber et al. did not find any survival benefit associated with LND when comparing 11,867 patients with non-metastatic RCC undergoing partial or radical nephrectomy with LND to a propensity-score matched cohort of patients who did not receive LND (OS 34.7 vs. 34.9 months, respectively)20. The NCDB has also been used to emulate the methods of EORTC 30881 using propensity score matching, with results showing no survival advantage of LND, even when adjusted to include a greater proportion of high-risk patients41.
The Role of Adjuvant and Perioperative Therapy in Node-Positive RCC
While nephrectomy is considered the gold standard treatment for non-metastatic RCC, up to 40% of patients may recur after an extirpative intervention45. Recurrence rates can be as high as 80% in those with node-positive disease, with 5-year survival as low as 11-35%46, 47. Thus, exploration of multimodal therapy is vital to addressing the shortcomings of nephrectomy and improving outcomes in node-positive RCC. Due to the significant risk of progression to metastatic disease, patients with node-positive RCC are prime candidates for early intervention33.
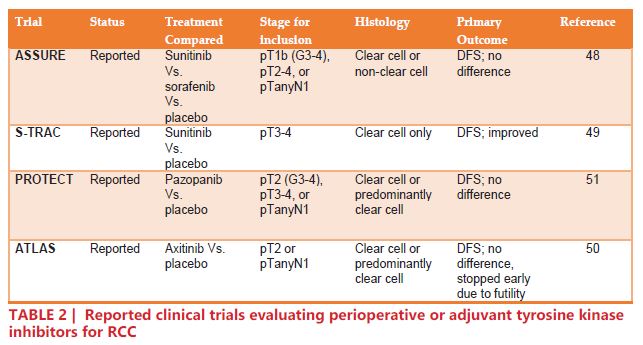
Thus far, four adjuvant trials that included patients with node-positive RCC have reported their results (TABLE 2). The ASSURE trial randomized 1,943 patients with completely resected pT1b, pT2-4, or TanyN+ RCC to one of three arms: sunitinib, sorafenib, or placebo, for 54 weeks48. The analysis showed no difference in disease free survival (DFS) for either sunitinib or sorafenib compared to placebo (HR 1.02, 97.5% CI 0.85 – 1.23; p=0.8038 and HR 0.97, 97.5% CI 0.80 – 1.17; p=0.7184, respectively)48. The S-TRAC trial randomized 615 pT3-4 or TanyN+ to sunitinib or placebo. The results of S-TRAC were more encouraging than those of ASSURE, concluding that patients in the sunitinib arm had significantly longer DFS compared to placebo (HR 0.76, 95% CI 0.59 – 0.98; p=0.03)49. The ATLAS trial randomized 724 patients with previously-resected RCC (≥pT2 and/or N+) to axitinib or placebo. On the primary analysis of DFS, there was no significant difference in the intention-to-treat population (HR 0.87, 95% CI 0.660 – 1.147; p=0.3211)50. The PROTECT trial randomized 1,538 patients with pT2, pT3, and pT4 disease to pazopanib or placebo. Initially, the dose was set at 800 mg daily, but was later reduced to 600 mg due to significant adverse effects. Interestingly, while the 600 mg group showed no significant reduction in DFS (HR 0.86, 95% CI 0.70 – 1.06; p = 0.165), the 800 mg group did (HR 0.69, 95% CI 0.51 – 0.94; p=0.02)51.
Akin to the prior efforts to orchestrate immune-mediated antineoplastic activity through cytokines, checkpoint inhibitors have come to the forefront as a promising therapeutic option for metastatic RCC. In the Checkmate 025 trial, the checkpoint inhibitor nivolumab showed significant improvement in OS with fewer adverse effects when compared to everolimus (HR of death 0.73, 98.5% CI 0.59 – 0.93; p=0.002)52. Checkmate 214, the landmark phase III trial that compared nivolumab plus ipilimumab versus sunitinib in metastatic RCC, demonstrated improved complete response rate (9% vs 1%) and improved OS for the checkpoint inhibitor arm (HR 0.63, 99.8% CI 0.44-0.89, p<0.001)53. Given the success of these agents in the management of metastatic RCC, integrating these therapies as adjuvant therapies may be a logical next step for patients at high-risk for metastatic progression, such as node-positive RCC. However, to date there have been no reported results from trials examining the role of checkpoint inhibitors as adjuvant therapy.
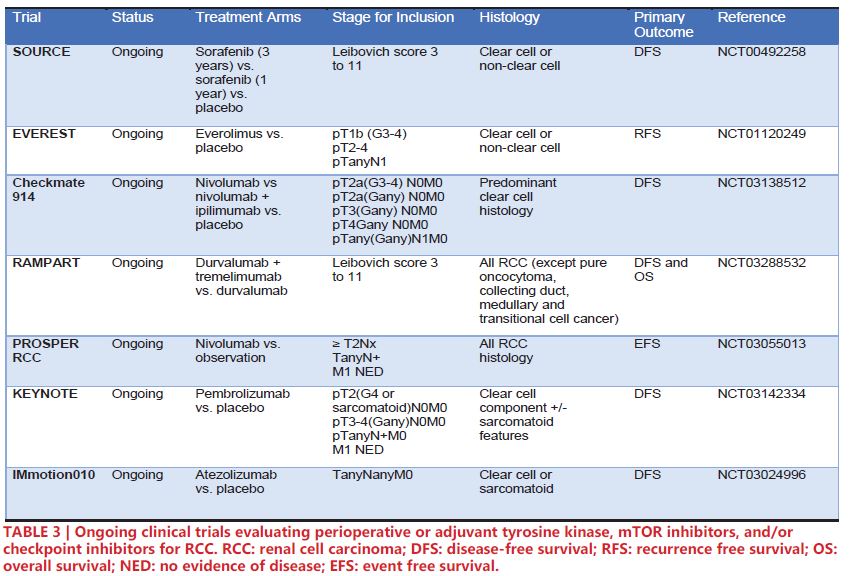
Noteworthy ongoing phase III trials for perioperative/adjuvant therapy are highlighted in Table 3. SORCE (NCT00492258) is an ongoing trial comparing sorafenib 3 years vs. sorafenib 1 year vs. placebo. However, preliminary results presented at European Society for Medical Oncology 2019 showed no significant increase in DFS for patients in the sorafenib arms54. Similarly, EVEREST (NCT01120249) is an ongoing clinical trial investigating the potential of the mTOR inhibitor everolimus. The recent success of Checkpoint 025 and Checkpoint 214 in demonstrating clinical utility of nivolumab and ipilimumab for RCC has led to five ongoing phase III clinical trials to implement checkpoint inhibitors in the adjuvant/ perioperative space: Checkmate 914 (NCT03138512) – nivolumab plus ipilimumab vs. versus nivolumab vs. placebo, RAMPART (NCT03288532) – durvalumab plus tremelimumab vs. durvalumab vs. observation, PROSPER RCC (NCT03055013) – perioperative nivolumab vs. observation, KEYNOTE (NCT03142334) – pembrolizumab vs. observation, and IMmotion010 (NCT03024996) – atezolizumab vs. observation. Notably, PROSPER RCC incorporates a neoadjuvant aspect, potentially allowing for translational studies of tissue and sera by comparing pre- and post-nivolumab treated tissue55.
There is a significant need to address the limitations of nephrectomy and LND in node-positive RCC. However, there is a dearth of evidence to direct the therapy for those with nodal disease. While 5%-47% of the patient population in the aforementioned trials – ASSURE, S-TRAC, ATLAS, PROTECT, Checkmate 025 and Checkmate 214 – were nodepositive, no study completed a subgroup analysis in this population of interest48-53. It is imperative that investigation into this unique population is included in future trials exploring the role of systemic therapies in the treatment of locally advanced and metastatic RCC.
CONCLUSIONS
The presence of pathologic lymph nodes in patients with non-metastatic kidney cancer has crucial prognostic value. Outcomes from several recent studies suggest that revising staging categories may lead to improved prognostication for patients with advanced RCC and have implications for therapy selection and clinical trial participation. It remains unclear whether LND can be a beneficial surgical option for a select subset of patients with RCC. Much of this uncertainty stems from a lack of level one evidence regarding nodal disease in RCC. However, with several ongoing and upcoming clinical trials that include patients with node-positive RCC, anticipated results may lead to a paradigm shift in the management of this disease. It is imperative that physicians work to enroll patients in clinical trials in order to gain a better understanding of the complexities of this disease, and ultimately improve the care of our patients.
REFERENCES
- 1. Siegel, R.L., K.D. Miller, and A. Jemal, Cancer statistics, 2020. CA: A Cancer Journal for Clinicians, 2020. 70(1): p. 7-30.
- 2. Amin, M.B., Edge, S., Greene, F., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., Jessup, J.M., Brierley, J.D., Gaspar, L.E., Schilsky, R.L., Balch, C.M., Winchester, D.P., Asare, E.A., Madera, M., Gress, D.M., Meyer, L.R., AJCC Cancer Staging Manual. 8th ed. 2017: Springer. 1032.
- 3. Messing, E.M., et al., Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol, 2006. 7(6): p. 472-9.
- 4. Pak, S., et al., Adjuvant chemotherapy versus observation after radical cystectomy in patients with node-positive bladder cancer. Sci Rep, 2019. 9(1): p. 8305.
- 5. Boström, P.J., et al., Benefit of Adjuvant Chemotherapy and Pelvic Lymph Node Dissection in pT3 and Node Positive Bladder Cancer Patients Treated with Radical Cystectomy. Bladder Cancer, 2016. 2(2): p. 263-272.
- 6. Leow, J.J., et al., Adjuvant Chemotherapy for Invasive Bladder Cancer: A 2013 Updated Systematic Review and Meta-Analysis of Randomized Trials. European Urology, 2014. 66(1): p. 42-54.
- 7. Williams, S.D., et al., Immediate Adjuvant Chemotherapy versus Observation with Treatment at Relapse in Pathological Stage II Testicular Cancer. New England Journal of Medicine, 1987. 317(23): p. 1433-1438.
- 8. Campbell, S., et al., Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol, 2017. 198(3): p. 520-529.
- 9. Network, N., NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines™) Kidney Cancer. 2016, Version.
- 10. Assouad, J., et al., Renal lymphatic drainage and thoracic duct connections: implications for cancer spread. Lymphology, 2006. 39(1): p. 26-32.
- 11. Crispen, P.L., et al., Lymph Node Dissection at the Time of Radical Nephrectomy for High-Risk Clear Cell Renal Cell Carcinoma: Indications and Recommendations for Surgical Templates. European Urology, 2011. 59(1): p.18-23.
- 12. Herrlinger, A., et al., Results of 381 transabdominal radical nephrectomies for renal cell carcinoma with partial and complete en-bloc lymph-node dissection. World Journal of Urology, 1984. 2(2): p. 114-121.
- 13. Pantuck, A.J., et al., Renal cell carcinoma with retroperitoneal lymph nodes: role of lymph node dissection. J Urol, 2003. 169(6): p. 2076-83.
- 14. Blom, J.H., et al., Radical nephrectomy with and without lymph-node dissection: final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol, 2009. 55(1): p. 28-34.
- 15. John, N.T., K.A. Blum, and A.A. Hakimi, Role of lymph node dissection in renal cell cancer. Urol Oncol, 2019. 37(3): p. 187-192.
- 16. Kates, M., et al., Decreasing Rates of Lymph Node Dissection During Radical Nephrectomy for Renal Cell Carcinoma. Annals of Surgical Oncology, 2012. 19(8): p. 2693-2699.
- 17. Capitanio, U. and B.C. Leibovich, The rationale and the role of lymph node dissection in renal cell carcinoma. World Journal of Urology, 2017. 35(4): p. 497- 506.
- 18. Patel, H.D., et al., Clinical Stage Migration and Survival for Renal Cell Carcinoma in the United States. European Urology Oncology, 2019. 2(4): p. 343-348.
- 19. Radadia, K.D., et al., Accuracy of clinical nodal staging and factors associated with receipt of lymph node dissection at the time of surgery for nonmetastatic renal cell carcinoma. Urol Oncol, 2019. 37(9): p. 577.e17-577.e25.
- 20. Farber, N.J., et al., Trends and outcomes of lymphadenectomy for nonmetastatic renal cell carcinoma: A propensity score-weighted analysis of the National Cancer Database. Urol Oncol, 2019. 37(1): p. 26-32.
- 21. Zareba, P., J.H. Pinthus, and P. Russo, The contemporary role of lymph node dissection in the management of renal cell carcinoma. Therapeutic advances in urology, 2018. 10(11): p. 335-342.
- 22. Campi, R., et al., Templates of Lymph Node Dissection for Renal Cell Carcinoma: A Systematic Review of the Literature. Frontiers in surgery, 2018. 5: p. 76-76.
- 23. Capitanio, U., et al., Lymphadenopathies in patients with renal cell carcinoma: clinical and pathological predictors of pathologically confirmed lymph node invasion. World J Urol, 2016. 34(8): p. 1139-45.
- 24. Gershman, B., et al., Radiographic size of retroperitoneal lymph nodes predicts pathological nodal involvement for patients with renal cell carcinoma: development of a risk prediction model. BJU Int, 2016. 118(5): p. 742-749.
- 25. Guimaraes, A.R., et al., Pilot study evaluating use of lymphotrophic nanoparticle- enhanced magnetic resonance imaging for assessing lymph nodes in renal cell cancer. Urology, 2008. 71(4): p. 708-12.
- 26. Kroeger, N., et al., Characterizing the impact of lymph node metastases on the survival outcome for metastatic renal cell carcinoma patients treated with targeted therapies. Eur Urol, 2015. 68(3): p. 506-15.
- 27. Capitanio, U., et al., Lymph node dissection in renal cell carcinoma. Eur Urol, 2011. 60(6): p. 1212-20.
- 28. Blute, M.L., et al., A protocol for performing extended lymph node dissection using primary tumor pathological features for patients treated with radical nephrectomy for clear cell renal cell carcinoma. J Urol, 2004. 172(2): p. 465-9.
- 29. Yu, K.J., et al., Renal cell carcinoma and pathologic nodal disease: Implications for American Joint Committee on Cancer staging. Cancer, 2018. 124(20): p.4023-4031.
- 30. Shao, N., et al., Modification of American Joint Committee on cancer prognostic groups for renal cell carcinoma. Cancer medicine, 2018. 7(11): p. 5431-5438.
- 31. Srivastava, A., et al., Impact of pathologic lymph node-positive renal cell carcinoma on survival in patients without metastasis: Evidence in support of expanding the definition of stage IV kidney cancer. Cancer, 2020. 126(13): p. 2991-3001.
- 32. Gershman, B., et al., Renal Cell Carcinoma with Isolated Lymph Node Involvement: Long-term Natural History and Predictors of Oncologic Outcomes Following Surgical Resection. European Urology, 2017. 72(2): p. 300-306.
- 33. Shapiro, D.D. and E.J. Abel, Renal cell carcinoma with isolated lymph node metastases should be reclassified as stage IV. Cancer, 2020. 126(13): p. 2965- 2967.
- 34. Patel, H.V., et al., Strengthening the foundation of kidney cancer treatment and research: revising the AJCC staging system. Annals of Translational Medicine, 2019: p. 33.
- 35. Patel, H.V., A. Srivastava, and E.A. Singer, To Be or "Node" to Be: Nodal Disease and the Role of Lymphadenectomy in the Treatment of Renal Cell Carcinoma. Medical research archives, 2020. 8(5): p. 2091.
- 36. Herrlinger, A., et al., What are the benefits of extended dissection of the regional renal lymph nodes in the therapy of renal cell carcinoma. J Urol, 1991. 146(5): p. 1224-7.
- 37. Schafhauser, W., et al., Lymph node involvement in renal cell carcinoma and survival chance by systematic lymphadenectomy. Anticancer Res, 1999. 19(2c): p. 1573-8.
- 38. Bhindi, B., et al., The role of lymph node dissection in the management of renal cell carcinoma: a systematic review and meta-analysis. BJU Int, 2018. 121(5): p. 684-698.
- 39. Studer, U.E. and F.D. Birkhäuser, Lymphadenectomy combined with radical nephrectomy: to do or not to do? Eur Urol, 2009. 55(1): p. 35-7.
- 40. Capitanio, U., et al., Stage-specific effect of nodal metastases on survival in patients with non-metastatic renal cell carcinoma. BJU International, 2009. 103(1): p. 33-37.
- 41. Bacic, J., et al., Emulating Target Clinical Trials of Radical Nephrectomy With or Without Lymph Node Dissection for Renal Cell Carcinoma. Urology, 2020. 140: p. 98-106.
- 42. Gershman, B., et al., Lymph Node Dissection is Not Associated with Improved Survival among Patients Undergoing Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma: A Propensity Score Based Analysis. Journal of Urology, 2017. 197(3 Part 1): p. 574-579.
- 43. Gershman, B., et al., Radical Nephrectomy With or Without Lymph Node Dissection for Nonmetastatic Renal Cell Carcinoma: A Propensity Score-based Analysis. European Urology, 2017. 71(4): p. 560-567.
- 44. Feuerstein, M.A., et al., Analysis of lymph node dissection in patients with ≥7-cm renal tumors. World Journal of Urology, 2014. 32(6): p. 1531-1536.
- 45. Chin, A.I., et al., Surveillance strategies for renal cell carcinoma patients following nephrectomy. Rev Urol, 2006. 8(1): p. 1-7.
- 46. Babaian, K.N., et al., Preoperative Predictors of Pathological Lymph Node Metastasis in Patients with Renal Cell Carcinoma Undergoing Retroperitoneal Lymph Node Dissection. Journal of Urology, 2015. 193(4): p. 1101-1107.
- 47. Smaldone, M.C., et al., Adjuvant and neoadjuvant therapies in high-risk renal cell carcinoma. Hematol Oncol Clin North Am, 2011. 25(4): p. 765-91.
- 48. Haas, N.B., et al., Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet, 2016. 387(10032): p. 2008-16.
- 49. Ravaud, A., et al., Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med, 2016. 375(23): p. 2246-2254.
- 50. Gross-Goupil, M., et al., Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann Oncol, 2018. 29(12): p. 2371-2378.
- 51. Motzer, R.J., et al., Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma. J Clin Oncol, 2017. 35(35): p. 3916-3923.
- 52. Motzer, R.J., et al., Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. New England Journal of Medicine, 2015. 373(19): p. 1803-1813.
- 53. Motzer, R.J., et al., Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. New England Journal of Medicine, 2018. 378(14): p. 1277-1290.
- 54. T.Q.G. Eisen, E.F., B. Smith, A. Ritchie, R.S. Kaplan, B. Oza, I.D. Davis, M.R. Stockler, L. Albiges, B. Escudier, J.M.G. Larkin, A. Bex, S. Joniau, B.W. Hancock, G.G. Hermann, J. Bellmunt, E. Hodgkinson, P. Hanlon, M.K.B. Parmar, A.M. Meade, 2483 - Primary Efficacy analysis results from the SORCE trial (RE05): Adjuvant sorafenib for renal cell carcinoma at intermediate or high risk of relapse: an international, randomised double-blind phase III trial led by the MRC CTU at UCL. Annals of Oncology, 2019. 30: p. v851-v934.
- 55. Patel, H.D., et al., The future of perioperative therapy in advanced renal cell carcinoma: how can we PROSPER? Future Oncol, 2019. 15(15): p. 1683-1695.