ROUNDTABLE DISCUSSION
TKIs Beyond Second-Line Therapy: New Perspectives in Renal Cell Carcinoma Therapeutics
¹Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN; 2Charles A. Sammons Cancer Center, Texas AM College of Medicine, Dallas, TX; 3Cedars-Sinai Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Health System, Los Angeles, CAABSTRACT
This roundtable discussion held on March 10, 2021 explores the potential role of current tyrosine kinase inhibitors (TKIs) in the therapeutic landscape of advanced renal cell carcinoma (aRCC). This discussion also integrates new concepts emerging from a phase-3 TIVO-3 trial which demonstrated a robust safety/tolerability portfolio of a novel drug tivozanib as third- or fourth-line therapy for patients with heavily pretreated aRCC while preserving the quality of life (QoL) of these patients
Webinar Transcript
Dr. Figlin:
Welcome to the Kidney Cancer Journal webinar, focusing on exciting developments in renal cancer therapeutics. I am Robert A Figlin, Steven Spielberg Family Chair in Hematology-Oncology, at Cedars Sinai Medical Center in Los Angeles. I am going to moderate this session with my colleagues Drs. Brian Rini and Thomas Hutson. As many of you know, Brian is an Ingram Professor of Medicine and leads kidney cancer clinical research efforts at Vanderbilt-Ingram Cancer Center. Dr. Thomas Hutson, well known to all of you, is the director of the Urologic Oncology Program, and co-chair of the Urologic Cancer Research and Treatment Center at Baylor University, and Professor of Medicine at Texas A&M College. This is an interesting time and we are going to focus on a novel drug tivozanib, which on March 10, was approved by the FDA for advanced or refractory kidney cancer, after second line therapies1. Let's start with Brian (Rini). Can you please talk about the tivozanib molecule and especially its potential role in targeting VEGF receptors?
Dr. Rini:
In the family of TKIs, you have more selective agents like tivozanib and axitinib and you have multi-targeting agents - sorafenib and cabozantinib. The beauty of tivozanib is its selectivity and potency against the VEGFR targets and, as you all know that is integral to the biology of kidney cancer and fundamental to its very being. Which is why these VEGF inhibitors have precise activities2. Tivozanib was developed to be a potent and selective agent2,3 which I think probably is mostly reflected in its tolerability profile, so we do not see off-target toxicities with tivozanib, and you just tend to see on-target side effects like hypertension etc.

Thomas (Hutson), I always like having you on the call because of your pharmacy background. In terms of pharmacology and pharmacodynamics, how should a practicing medical oncologist think about tivozanib when using and delivering it in a clinical setting?10,11.
Dr. Hutson:
What is really striking about the tivozanib molecule is that at nanomolar concentration, it can inhibit VEGF receptors 1, 2, and 3, which are the putative receptors known to be important in kidney cancer pathogenesis2 and equally, it does not inhibit the off-target receptors like c-Kit, which contribute to side effects. Pharmacodynamically it is very potent. Pharmacokinetically, tivozanib has a half-life of 99 hours so it is going to stay in the system for a longer period of time. Although tivozanib is similar to axitinib in terms of specificity, it has a longer half-life than axitinib. Some investigators believe that this long half-life may be advantageous. Certainly, the pharmacokinetic and pharmacodynamic profile of tivozanib allows for very small milligram dosing, and it is given for three weeks on and one week off allowing for continual suppression of VEGF receptor. Overall, this results in better tolerability and then the prolonged suppression of VEGF receptor4.
Dr. Figlin::Yes, I think that is very insightful because when we are treating patients, we think about not only the target, but also we think about the half-life of the molecules to see if we need to hold or discontinue depending upon their toxicity profiles. So Brian, next take us through tivozanib’s development, a little bit about TIVO-15 and more recently, TIVO-3 clinical trial6 that ultimately has led to FDA approval. So help us understand the patient population, some of the results and dive into the outcomes that you think are important.
Dr. Rini:
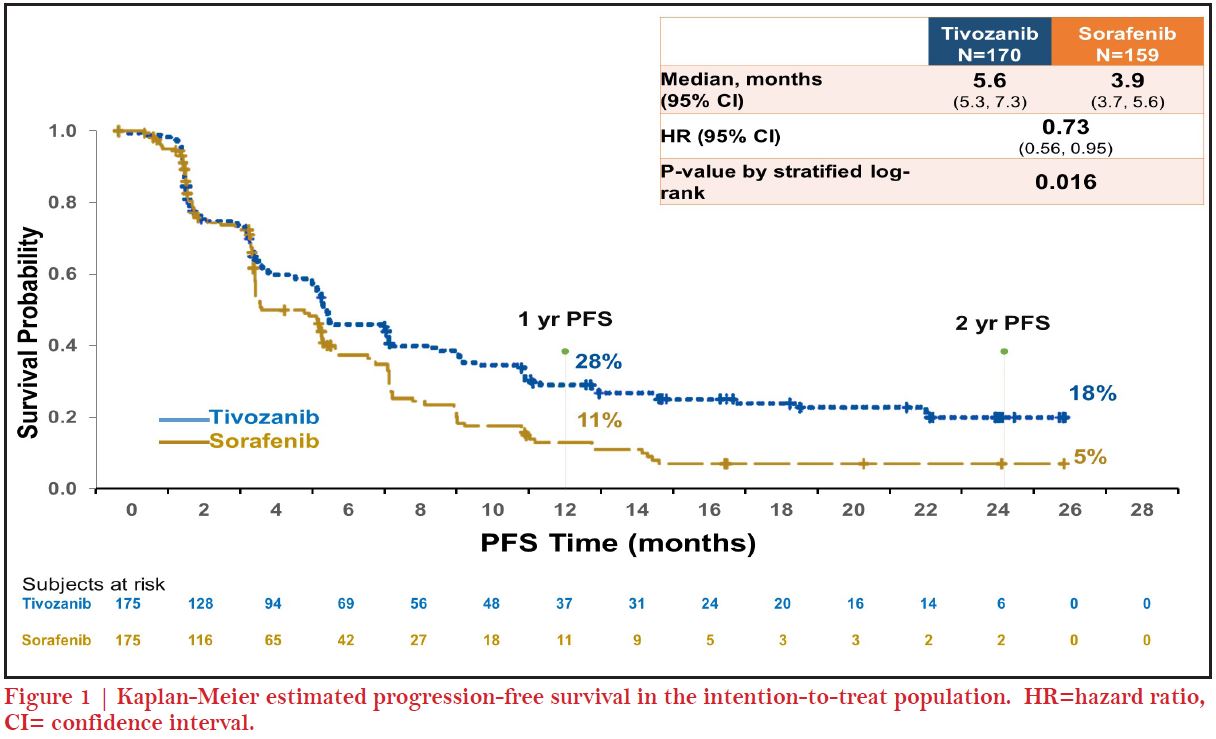
Sure, as we were discussing, tivozanib probably has one of the most interesting regulatory and development histories for an anti-cancer molecule. This is probably going back ten years, there was an initial phase-2 study published at the ASCO meeting3, 4 and it came along at least in a 2nd wave of TKI development. TIVO-1 was a large phase-3 study in the frontline setting, involving previously untreated patients randomized to tivozanib vs sorafenib5. Tivozanib had its progression free survival (PFS) endpoint and response rate (RR) advantages and tivozanib was very potent as other TKIs in the frontline setting5. However, the problem with TIVO- 1 was its one-way crossover design; when patients progressed on sorafenib, they crossed over and got tivozanib, which we now know is a very potent refractory agent. Whereas some patients who were initially randomized into tivozanib and did not cross over left to get a standard of care, which probably would not be a problem today but at the time and especially in the countries where it was conducted in parts of eastern Europe and Russia, there was no second line therapy so it became a trial of two drugs versus one; sorafenib + tivozanib versus tivozanib alone for many patients. Because of that, the survival hazard ratio was above one, which I believe really reflects that two drugs versus one drug phenomenon. But at the time, the FDA was not so convinced and certainly you can understand that they do not want to approve a drug that may adversely affect survival. Also, you can see how different regulators view data differently; the drug was approved in Europe years later, although it was not approved in the US7. TIVO-3 was eventually developed as a response by AVEO (clinicaltrials.gov, NCT02627963) to avoid this crossover problem6. So that is the reason why tivozanib was a bit unique in a refractory setting because you can no longer do frontline TKI versus frontline TKI. TIVO-3 trial showed PFS and ORR advantages in the later lines setting6. Some people have questioned the use of sorafenib as a control arm but that was entirely in response to TIVO-1 so as to recapitulate the study again in a different setting. We have seen that in other TKI trials on TKI versus TKI have shown about equivalent survival outcomes, reflection of all the active drugs that patients can get upon progression. So that is the very short version of a very long TIVO history.

Thomas, your thoughts about quality of life (QoL) data associated with targeted effectiveness of VEGF inhibition and less off-target toxicity?18,19
Dr. Hutson:
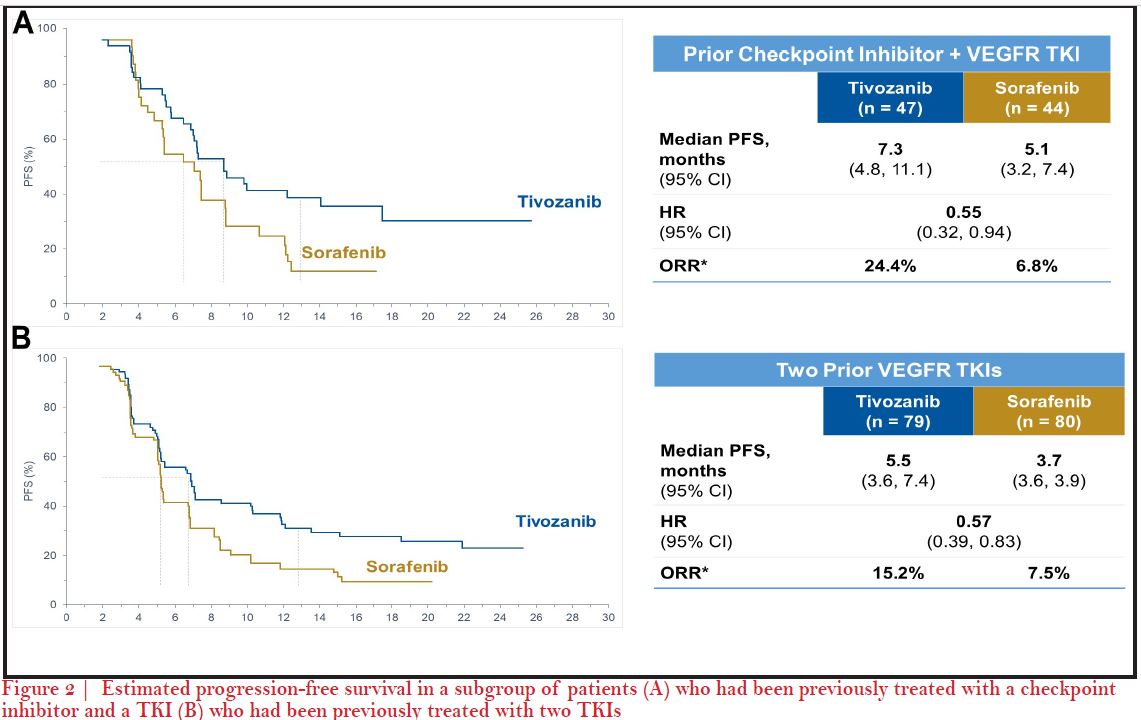
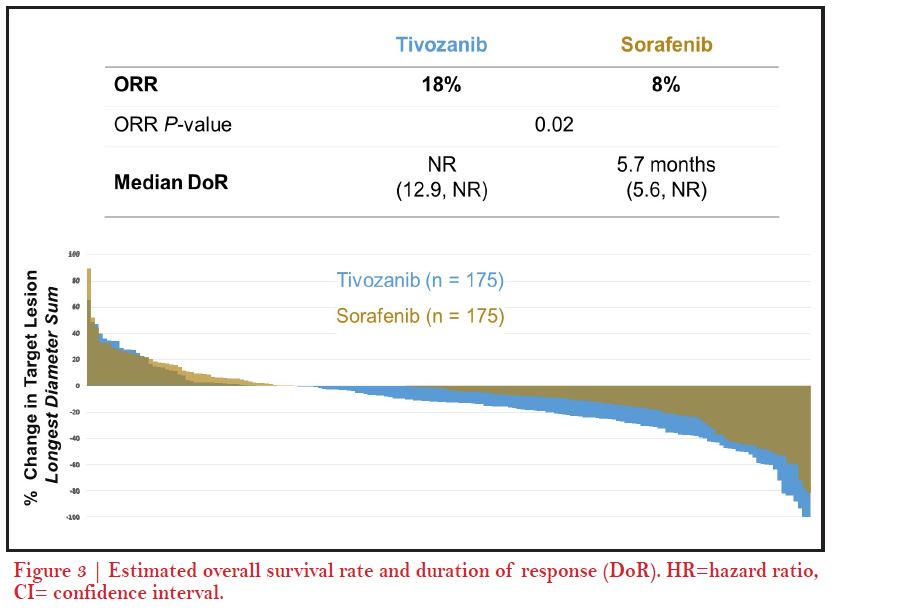
We saw the unique characteristics of tivozanib play out during its development from the phase-2 randomized discontinuation trial and we saw an untargeted and minimal level of grade 1 or 2 toxicities that have been problematic with this generation of TKIs4. For instance, some side effects like hand-foot skin reaction, fatigue were much less with tivozanib. We did see an increase in some side effects especially hypertension and dysphonia as a result of its potent inhibition of VEGFR4. Later, based on the results from phase-3 TIVO-1 trial where I was a senior author, we hoped for approval of tivozanib but unfortunately it was not approved in the US. The most recent trial of tivozanib, TIVO-3, really allowed us to reconfirm and shed light on the benefits of tivozanib and its tolerability in a refractory patient population which may not respond or tolerate therapy well6. So, what we know from this trial is that patients who have had prior VEGF targeted therapy like axitinib or prior IO therapies seem to have benefit efficacy, as well as good tolerability6. In particular, there was no sign of any new side effects and the side effects looked fairly similar to TIVO-1 study. A recent real world trial was published after tivozanib was approved in 2017 in the EU7. In a real world data analysis, our colleague Michael Staehler from the University of Munich, Germany, pulled together 23 patients between November, 2017 and October 2018, and treated patients both in the frontline setting as well as in second- line up to sixth line settings8. They were able to show what we had seen in the TIVO-1 and TIVO-3 trials that they were getting a median PFS of 14.9 months (95% CI 5.1–24.8). Median PFS was 30.3 months for first line patients versus 8.6 months (CI 5.1–12.2) (p=0.291) for later line which was again consistent with what we saw in Brian’s report. The side effects observed in terms of QoL were very similar to TIVO-3; hypertension, diarrhea, fatigue and hoarseness with grade one or two severity8.
Dr. Figlin::
Brian, this seems like evidence of VEGF dependence in kidney cancer and TKI therapy continues to benefit patients after multiple prior line therapies, even in later settings. So how do you conceptualize using this data in your day in, day out practice when you start seeing these patients post multiple prior lines, but still have some evidence of that VEGF dependence?
Dr. Rini:
Yes, I think your point is a good one, analogous to prostate cancer where it is still testosterone dependent through multiple lines of therapies, kidney cancers are dictated by VEGF through multiple lines of therapy. These patients in the third- or fourth- line settings had seen at least one VEGF therapy and perhaps some patients have seen two or more therapies. So you are absolutely right, the biology remains at least in part, although not in whole VEGF dependent that is why we see potent activity here. As you are aware, again, there is a debate - do you want to get more or less selective in your TKI use as you go into refractory setting? We could certainly argue that tivozanib is not necessarily a contemporary multi-targeting TKIs like cabozantinib or lenvatinib would be but, I think even more impressive when you have this level of potency with a very selective agent, because it specifically inhibits VEGF; not non-specific targets. So, again, to your point, there is a level of fundamental VEGF dependency here. To answer your question, as I move from an IO containing regimen upfront, I use a lot of IO-TKI to a refractory regimen which for me is usually a single agent TKI. My mindset has gone away from cure and is rather focused on disease control as I do not think TKIs cure patients as IO based therapy does. The tolerability profile of the agent has always been very important to me in the refractory setting. That is why I use a lot of axitinib in that setting9 before I was using axitinib-pembrolizumab10. So I think the major advantage for tivozanib is not just activity because I think the activity is probably comparable to other TKIs but also its tolerability. As patients get pretty beat up in the thirdand fourth- line settings, you are starting to question: Am I really helping this patient by giving them more therapy or am I hurting them more? I know this is something I face when patients are getting into third- or fourth- line setting so I am pretty careful about choosing agents with what I perceive the best tolerated profile. Because at least I am not harming the patient so I can use this agent very liberally in the third- or fourth- line setting or even if they fail to an IO- TKI regimen, I think tivozanib is perfectly appropriate in that setting.

Thomas, your thoughts on the potential of TKI therapies in the later line therapeutics space as an experienced investigator?
Dr. Hutson:
Yes, I agree with Brian. At a bit more granular level on the actual regimens we would choose IO-TKI in the community setting, especially axitinib based regimen, like axitinib-pembrolizumab is most utilized10. We are evaluating VEGF TKIs with IO therapies so you may have drugs combined to IO like cabozantinib or lenvatinib12, but when we start moving into the second line setting after cabozantinib and into the third line space, we know that lenvatinib- everolimus is a very active regimen. In the refractory setting, we are looking for a therapy as Brian communicated that can accomplish the goal of stabilizing disease. We are not looking so much at that shrinkage of tumor with the disease control rate which is actually very impressive if I recall, and then a tolerability profile that makes tivozanib an ideal drug to choose in a third line after a cabozantinib or a fourth line. So, again, what we showed in TIVO-3 was that you could have exposure to axitinib, as you would have in first line combo with an IO-TKI, and then later tivozanib, and still get this level of activity. Prior to TIVO-3 , we really did not have a lot of therapies with phase-3 data. But, now we know things are going to change as we know what you pick first, dictates what you choose second, third and fourth line. For instance, if you get cabozantinib-nivolumab13, that is going to change what you are going to get second as you are no longer going to get cabozantinib second, so have to think - could it be a tivozanib? axitinib, or could it be lenvatinib, everolumab? I think the data from TIVO-3 certainly makes tivozanib an ideal option in the later lines setting6.
Dr. Figlin::We know, for example that there is clearly a dose-response effect to TKIs targeting VEGFR in clear cell RCC. I am just wondering out loud to the two of you, whether the real benefits of tivozanib are in part explained by its nanomolar IC50 so that you can get such inhibition at relatively low concentrations?
Dr. Rini:
Yes, I think so. I am a big believer in an optimal dosing of TKIs and I spent a lot of time thinking about it. You can achieve the benefits with optimal dosing that is appealing to you in a clinical community practice. So I think there is good pharmacokinetics and pharmacodynamics. You have the halflife issue which could be good or could be bad. We can sort of debate that, but obviously it is what it is. I think the long half-life of tivozanib does not hurt patients because it is so darn tolerable due to its optimal dosing advantage. I think some other multi-target TKI agents are much more toxic in my opinion as it takes a long time to get out of the system. Therefore I just do not think there is any major tolerability issue to any extent with tivozanib even in later line setting.
Dr. Figlin::So you do not think that there is any challenge in navigating the hypertension associated with tivozanib because of the long half-life in terms of controlling it once a person develops it?
Dr. Rini:
I think in the early years we were all refreshing our memories about anti-hypertensives. But now it is been 15 or 20 years since we started dealing with with VEGF TKI associated hypertension or other side effects. So I feel my staff and I feel pretty comfortable managing hypertension. I can not think of a patient where I have permanently stopped for hypertension. As most people feel comfortable enough dealing with such issues, I do not think that is going to be a huge issue.
Dr. Figlin::For you Thomas?
Dr. Hutson:
Absolutely the same, there is no pure or ideal VEGF inhibitor. So what we see with tivozanib is that it is active even at nanomolar concentration, the next off-target is so much higher. You are just never going to get off-target toxicity from tivozanib as you would have to take a bottle of the drug at one time to hit other off-targets. We get only on-target side effects which are manageable, so I think that is what makes tivozanib so advantageous and well tolerated.
Dr. Figlin::So just thinking out loud, now that we have FDA approval for tivozanib, and we have good toxicity profile, do you think it is an easily combinable drug for future design, I mean is it something that we should be thinking about in clinical trial design involving next generation IO-TKI at a nanomolar concentration?
Dr. Hutson:
Yes, absolutely. We are looking for combinable therapies to add on the backbone of VEGF inhibitors. Since we know from the pathogenesis of clear cell renal carcinoma that VEGF is going to be an important target for us to continue to suppress, having a drug that has predictable side effects is going to be advantageous when we combine two. I think that is one of the advantages we have seen already in the marketplace with axitinib-pembrolizumab10 that it is gotten such great uptake as physicians feel the drug is well tolerated and I think they are going to be equally pleased when the tivozanib-nivolumab14 study continues to enroll and hopefully that will be a positive trial.
Dr. Figlin::You guys have been spectacular as I knew you would be, Brian and Tom. Why don't you speak to the community physician seeing the occasional clear cell RCC patient and kind of summarize for them, how they should be thinking about tivozanib and integrating it into their practice?
Dr. Rini:
I would think about it as a very clean, potent and well tolerated VEGF inhibitor and would integrate it early in the refractory setting, which is where the data supports. We will investigate Thomas's point about other combos and triplets as well. You will be pleasantly surprised not just at its efficacy, which I think is impressive but also at its tolerability especially after being beat up with a frontline doublet, or a second line combo. So, tolerability is the calling card of this tivozanib agent and I think you and your staff are going to like that very much.


Any special population data that we are aware of what happens in a brain metastatic patient? Is there any information from the TIVO-3 trial that helps us figure out exactly what kind of refractory patient might benefit?
Dr. Rini:
The short answer is no, I do not think brain mets were allowed and I do not think we have looked at organ subsets yet. You know those analyses are always a bit flawed and I am not aware of any data that would support a subpopulation that is particularly enriched or not enriched.
Dr. Figlin::Thomas, speaking to the community practice what would be your take home lessons?
Dr. Hutson:
Sure. For the community oncologist, I would also echo what Brian said that this would be one of the agents that you put in the tool box of therapies that you are going to choose from to give your patients. We now have the advantage or disadvantages of having multiple lines of therapy to choose from, knowing that patients never make it past the third or fourth line for most people. When treating a patient, it will be important to select the most active sequence of agents to make sure that patients are able to be exposed to the best therapies available. Having new therapies with data in later lines is crucial, therapies especially which provide disease control. So, tivozanib is going to be pushed over into that box of therapies we want to use. Unfortunately many patients do not make it past the fourth line of therapy and people need to realize this is the therapy they are going to want to have on their list of therapies to choose from.
Dr. Figlin::Well, Brian and Thomas, you have been spectacular as I expected you would be. This is a great summary of another novel agent that is going to have a potential role in treating our patients. Thank you and best regards.
REFERENCES
- 1. FDA approves tivozanib for relapsed or refractory advanced renal cell carcinoma. Drug Approvals and Database 2021 (March 10, 2021). https://www.fda.gov/drugs/drug-approvals- and-databases/fda-approves-tivozanib-relapsed- or-refractory-advanced-renal-cell-carcinoma .
- 2. Winston W et al. Tivozanib, a selective VEGFR TKI, potently blocks angiogenesis and growth in tumors that express a high level of VEGF-C and are refractory to VEGF-A blockade. AACR–NCI– EORTC International Conference: Molecular Targets and Cancer Therapeutics; San Francisco, CA; November 12–16, 2011.
- 3. Bhargava P, Esteves B, Al-Adhami M, Nosov DA, Lipatov ON, Lyulko AA, et al. Activity of tivozanib (AV-951) in patients with renal cell carcinoma (RCC): Subgroup analysis from a phase II randomized discontinuation trial (RDT). Journal of Clinical Oncology 28(15)_suppl (May 20, 2010) 4599-4599.
- 4. Nosov D, Bhargava P, Esteves WB, Strahs AL, Lipatov ON, Lyulko OO, et al. Final analysis of the phase II randomized discontinuation trial (RDT) of tivozanib (AV-951) versus placebo in patients with renal cell carcinoma (RCC). 2011 ASCO Meeting; 29(15_suppl): 4550.
- 5. Motzer RJ, Nosov D, Eisen T, and Hutson TE. Tivozanib Versus Sorafenib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma: Results From a Phase III Trial. Journal of Clinical Oncology 2013; 31(30), 3791-3799
- 6. Rini BI, Pal SK, Escudier BJ, Atkins MB, Hutson TE, Porta C, Verzoni E, Needle MN, McDermott DF. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020 Jan;21(1):95-104. PMID: 31810797.
- 7. European Medicines Agency E. Summary of Opiinion Fotivda, EMA/CHMP/333095/2017; Vol 2019. https://www.ema.europa.eu/en/documents/ smop-initial/chmp-summary-positive- opinion-fotivda_en.pdf.
- 8. Staehler M, Spek AK, Rodler S. Real-World Results from One Year of Therapy with Tivozanib. Kidney Cancer, 2019; 3 (4), 235-239. Journal of Clinical Oncology. 2019;3:541–541.
- 9. Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial Lancet. 2011 Dec 3; 378(9807):1931-9.
- 10. Motzer RJ , Penkov K , Haanen J , et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma, N Engl J Med. 2019. N Engl J Med 2019; 380:1103-1115.
- 11. Rini, BI, Plimack ER. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019; 380:1116-1127. 12. Motzer R, et al "Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma" N Engl J Med 2021; DOI: 10.1056/ NEJMoa2035716.
- 13. Choueiri TK, Powles T, Burotto M, et al. Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: first results from the randomized phase 3 CheckMate 9ER trial. Ann Oncol. 2020;31(4):S1159.
- 14. TiNivo: Safety and Efficacy of L Albiges, P Barthélémy, M Gross-Goupil, S Negrier, MN Needle, B Escudier. Tivozanib-Nivolumab Combination Therapy in Patients With Metastatic Renal Cell Carcinoma. Ann. Oncol. 2021. 32(1), 97-102.

Acknowledgments:
This article is supported
in part through independent
funding from

Declaration of interests:
BIR has served as a consultant to Arrowhead and received research funding from Peloton and research funding and honoraria from AstraZeneca, AVEO Oncology, Pfizer, Bristol-Myers Squibb, Roche, and Merck. TEH has served as an advisor to Pfizer, Exelexis, Bristol-Myers Squibb, AVEO Oncology, and Janssen. RAF has no relevant financial relationships with commercial interest to disclose pertaining to this article.

Disclosure:
The roundtable participants (authors) were invited to participate in this discussion by the journal. This article was peer-reviewed and the final content and article is the sole work of the authors.