Mechanistic Insights into the Obesity Paradox and Implications for Therapy
ABSTRACT
Exciting progress has been made towards uncovering mechanisms which underlie the paradoxical impact of obesity in renal cell carcinoma, suggesting new treatment strategies in this patient population. Enhancing our understanding of the complex interactions within the tumor microenvironment may allow us to identify novel targets for therapeutic action.
INTRODUCTION
The obesity epidemic has unfortunately only continued to worsen in the United States and across the globe. Obesity is determined by measuring one’s body mass index (BMI), weight divided by height squared. For adults, several weight classes have been defined, including normal weight (18.5 to 25 kg/ m2), overweight (>25 to <30 kg/m2), and class I through III obesity (30 to <35 kg/m2, 35 to <40 kg/m2, and >40 kg/m2, respectively). Trends suggest that approximately 1 in 2 adults will be obese in the United States in the year 2030, and severe obesity will become the most common weight class amongst women and low-income earning adults.1 With thirteen cancers linked with obesity2 and expenditures for obesity-related cancers accounting for an adjusted two-fold higher cost compared with non-obese cancers,3 understanding the biological impact of obesity and leveraging these insights to improve outcomes is paramount.
Clinical Insights into the Obesity Paradox
As in other malignancies, obesity remains a significant independent risk factor for the development of renal cell carcinoma (RCC).4-6 This risk remains weight dependent, as an incremental increase in BMI by 1 (kg/m2) is associated with a rise in risk of RCC by 4%.5 Uniquely, though, several independent reports have demonstrated an inverse association between BMI and mortality, termed the “obesity paradox” – obese patients have more favorable outcomes when compared to their non-obese counterparts. While many have argued that this trend may be due to confounding factors and analytic biases, other reports which have attempted to overcome these biases demonstrate this protective trend.7 In the localized setting, a recent meta-analysis of >1,500 patients showed a superior overall survival (OS) for patients with clear cell RCC who were overweight and obese (HR 0.57, 95% CI 0.43-0.76).8 Reported disease-specific patterns support this trend, as obesity has been linked to a lower risk of lymph node metastasis,9 and other factors associated with poorer prognosis, such as renal vein invasion, have been associated in male patients with lower BMI.10 Several independent reports confirm this protective trend in metastatic clear cell RCC,11-15 and preliminary integration of BMI into validated risk models like the International Metastatic RCC Database Consortium (IMDC) model has yielded several insights on potentially improving predictive performance.16 While prospective validation of tools like this is needed, the ease of computing a patient’s BMI during a clinical examination presents an attractive modifier that can likely be rapidly adopted in clinical practice.
Several reports have investigated the predictive and prognostic impact of obesity in patients with advanced clear cell RCC treated with systemic agents. In the seminal work by Albiges et al. investigators illustrated these trends in two large cohorts of patients treated with VEGFR-targeted tyrosine kinase inhibitors (TKIs) and mTOR inhibitors in the first-line and second-line settings.17 Patients from the curated IMDC database and a pooled validation cohort consisting of cases from prospective clinical trials were used for data analysis, totaling an impressive 4,657 patients for study. Patients were stratified based upon BMI ≥25 kg/m2 (which includes both overweight and obese patients), and patients with high BMI were found to have a superior OS (adjusted HR = 0.84, 95% CI = 0.74-0.93).17 Notably, this association was present in patients with IMDC intermediate- and poor-risk disease but did not reach significance in the favorable-risk population.
As obesity is characterized as a chronic, pro-inflammatory state, there has been a shift in focus to understanding the impact of BMI within the immune checkpoint inhibitor (ICI) era. McQuade et al. demonstrated a clinical association between BMI and ICI therapy outcomes in patients with advanced melanoma, with a superior response and survival in obese men compared with normal weight men.18 Interestingly, this protective association was not seen in female melanoma patients. For patients with advanced RCC, a single institutional cohort of metastatic RCC patients treated with immunotherapy at Memorial Sloan Kettering Cancer Center demonstrated that obesity was associated with superior clinical outcomes, but this did not appear to be independent of IMDC risk.19 In a multi-institutional cohort, RCC patients with high BMI were found to have improved progression-free survival (PFS) and superior overall survival (OS).20 This trend remained significant only in the overweight (25-30 kg/m2) but not the obese group. Pan-cancer studies that include metastatic RCC patients highlight this across tumors treated with ICI.21,22 As a corollary to these findings, as patients who benefit from ICI therapies are more likely to experience immune related adverse events (irAEs),23 a meta-analysis demonstrated that obesity was associated with an increased risk for irAE development across multiple tumor types.24
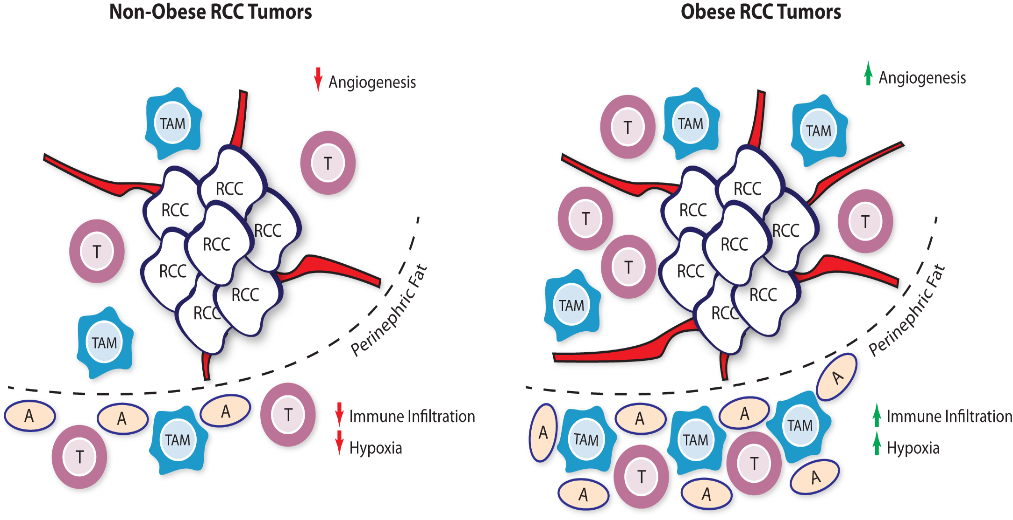 Fig. 1 | Obese and Non-Obese Renal Cell Carcinoma Tumor Microenvironment. RCC tumors from obese patients were found to have increased angiogenesis signatures which may promote response to VEGFR tyrosine kinase inhibitor therapy. The peritumoral fat tissue in obese RCC patients, compared to normal weight patients, also harbors increased hypoxia and immune cell infiltration signatures. These immune cells may act as a reservoir for infiltration upon checkpoint inhibitor therapy. RCC: renal cell carcinoma ; TAM: tumor-associated macrophage; T: T cell; A: Adipocyte.
Fig. 1 | Obese and Non-Obese Renal Cell Carcinoma Tumor Microenvironment. RCC tumors from obese patients were found to have increased angiogenesis signatures which may promote response to VEGFR tyrosine kinase inhibitor therapy. The peritumoral fat tissue in obese RCC patients, compared to normal weight patients, also harbors increased hypoxia and immune cell infiltration signatures. These immune cells may act as a reservoir for infiltration upon checkpoint inhibitor therapy. RCC: renal cell carcinoma ; TAM: tumor-associated macrophage; T: T cell; A: Adipocyte.
With the introduction of VEGFR TKI and ICI combinatorial strategies, it remains to be seen whether these trends persist with the use of both VEGFR TKI and ICI therapies together. In the subgroup analysis of the randomized phase III study of axitinib plus avelumab, superiority over sunitinib was found regardless of BMI status.25 Efforts to characterize the impact of novel targeted agents with ICI therapies in this context to identify if trends remain consistent underway.
Obesity and Renal Cell Carcinoma – Mechanistic Insights
With multifactorial metabolic and immune-regulated changes associated with obesity, it has been challenging to decipher the specific mechanisms which may underlie this paradoxical trend in patients with advanced RCC. Several questions remain — does obesity lead to change in natural immunity which impairs tumor surveillance or does obesity contribute to the evolution of a distinct tumoral phenotype? Prior research has demonstrated that obesity may activate mTOR and hypoxia-inducible factor-1 (HIF-1) signaling in immune cells,26 highlighting overlapping pathways pertinent in clear cell RCC tumorigenesis. With more longitudinal studies focused on uncovering cellular mechanisms related to obesity, we can better link disease pathophysiology with these clinical trends.
In the search for differences in RCC tumor phenotypes in the obese setting, several investigators have looked to specific mutational or gene-expression profiles from obese and normal weight patients. RCC-specific alterations like VHL and PBRM1 have been shown to occur in similar frequencies in these two groups.27 Molecular subtyping by ClearCode34 a gene-expression profiling tool, highlights that obese and diabetic patients are more likely to harbor clear cell type A tumors characterized by angiogenesis dominant profiles.28 Translational work using the Cancer Genome Atlas (TCGA) dataset also found that tumoral expression of fatty acid synthase (FASN), a key enzyme involved in lipogenesis and the production of long-chain fatty acids, correlates with BMI in clear cell RCC patients.17 In that study, investigators found that patients with low FASN tumors had superior OS, and high FASN expression was found more often in patients with IMDC poor-risk versus favorable-risk disease.17
Obesity’s effect on immune dysregulation has been well established and exploring these downstream consequences of obesity, particularly in RCC, has led to several interesting observations. In preclinical models of diet-induced obese (DIO) mice, widespread changes in cytokines and chemokines were seen in tumor-bearing mice compared to controls,29 and dendritic-cell (DC)-based immunotherapy was associated with faster tumor growth in obese mice. Therapeutic failure in this context was proposed to stem from an increase in immunosuppressive DCs within the tumor microenvironment (TME), and a lower influx of CD8+ effector T cells. Other reports have also detailed that DIO mice have higher levels of myeloid-derived suppressor cells (MDSCs), with elevated DCs and tumor-associated macrophages which traffic to the TME via CCL2.30 While obese patients with clear cell RCC have been shown to have fewer circulating PD-1+/CD8+ T cells, other circulating immune populations remain similar when compared to normal-weight patients.31
In follow up to the pivotal work by McQuade et al.,18 Wang and colleagues investigated potential mechanisms that may underlie the obesity paradox in relation to ICI therapy.32 Using melanoma and animal models and patient samples, investigators consistently found upregulation of PD-1 expression and low T cell proliferation in obese patients. Within the TME, isolated T cells had upregulation of immune checkpoint proteins including PD-1, Tim3, and Lag3 expression, and significant upregulation of specific T cell profiles associated with anergy and senescence. Interestingly, researchers found that PD-1 CD8+ expression correlated with elevated levels of leptin, a hormone commonly implicated in obesity. Utilizing a leptin-deficient tumor model, mice treated with leptin were found to have faster tumor growth and higher T cell exhausted phenotypes, suggesting the critical role leptin signaling may have in immunity and the role ICI therapy may have in restoring immune sensitivity with leptin-mediated dysregulation.32 Others have shown high levels of leptin in RCC tissues associated with a shorter PFS,33 but a direct link between leptin and T cell function in this context for patients with RCC remains unclear.
Given the differences in immune regulation that are seen in obese patients, Sanchez and colleagues investigated whether immune infiltration within the RCC tumor and peritumoral microenvironment might shed light into other components which may affect clinical outcomes (Figure 1).27 The investigators compiled three different data sets comprising of patients from COMPARZ, the randomized phase III study of sunitinib and pazopanib, TCGA, and an observational cohort of patients with metastatic clear cell RCC treated at Memorial Sloan Kettering Cancer Center. Using gene set enrichment profiling and immune deconvolution analysis, the degree and type of immune populations within each tissue sample was estimated for each of these tumors. Firstly, they confirmed the protective effect of obesity in this population compared to normal-weight patients in both the COMPARZ (all advanced metastatic) and TCGA (mostly early stage) cohorts (Figure 2). Interestingly, results with ICI therapy did not remain significant in regards to obesity when adjusted for IMDC risk. In the COMPARZ cohort, they performed transcriptional analysis and found that tumors from patients who were obese had significant upregulation of several pathways involved in hypoxia, angiogenesis, TGF-β signaling, and epithelial -mesenchymal transition, and upregulation of metabolic pathways like adipogenesis, fatty acid metabolism, and glycolysis. With these changes in angiogenesis within the microenvironment, the investigators propose that these changes may explain the increased sensitivity to VEGFR TKI therapy. They then focused their work on the tumor microenvironment and did not find a significant difference in the degree of total immune infiltration within the TME and found that tumors from patients who were obese harbored lower expression of immune checkpoint molecules. Extending their search towards the peritumoral fat, they did identify a significant difference in inflammatory signatures in these regions when compared to the peritumoral environment of tumors from normal-weight patients, with higher levels of classically activated M1 TAM phenotypes. With the degree of immune infiltration and immune sensitivity of RCC tumors, this study prominently underscores the importance of peritumoral signaling interactions and extended our view of the TME to surrounding tissue compartments.
Harnessing Obesity-Related Mechanisms for Future Discovery
While the reports discussed here lay the groundwork for understanding mechanistic differences related to obesity and immunity, translating these findings into clinical practice remain to be done. A common limitation often faced in many of these studies is that a single BMI data point may not reflect the longitudinal biological changes which occur during therapy. Hence, understanding whether other measurements related to obesity may also serve as surrogates for underlying physiologic changes in muscle, fat and metabolic fitness is vital. For instance, cross-sectional imaging can be used to calculate the skeletal muscle index (SMI) or skeletal muscle radiodensity (SMD), surrogates for body muscle mass, and a lower SMI at baseline has been associated with a shortened OS.34 SMI also appears to be prognostic in RCC patients treated with targeted agents.35 Other measures like subcutaneous fat have been shown to be highly correlated with BMI but not significantly associated with survival outcomes for RCC patients.36 With novel software acquisition tools which can automate capture of these different muscle and fat compartments, prospective studies which can track long-term physiologic changes related to metabolic syndrome may uncover novel insights with these clinical trends.
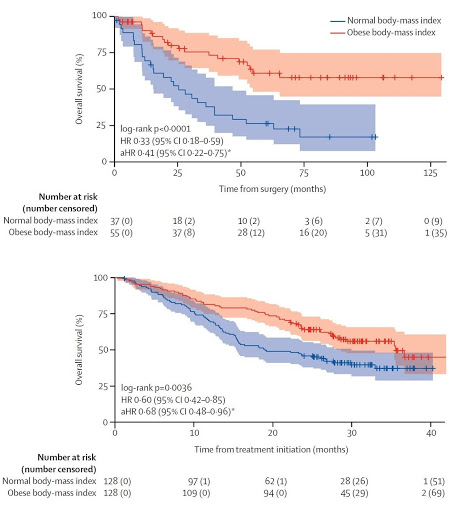 Fig. 2 | Overall Survival of Obese and Normal Weight clear cell RCC patients. Kaplan- Meier curves comparing obese and non-obese clear cell RCC patients from the TCGA (mostly early stage) (A) and COMPARZ (all advanced metastatic) (B) cohorts, showing obese patients have longer overall survival, and longer overall survival when treated with first-line VEGFR tyrosine kinase inhibitor therapy. Shaded areas are 95% confidence interval. [Figure adapted and modified from Sanchez A et al., Lancet Oncology, 21(2), 283-293, 2020].
Fig. 2 | Overall Survival of Obese and Normal Weight clear cell RCC patients. Kaplan- Meier curves comparing obese and non-obese clear cell RCC patients from the TCGA (mostly early stage) (A) and COMPARZ (all advanced metastatic) (B) cohorts, showing obese patients have longer overall survival, and longer overall survival when treated with first-line VEGFR tyrosine kinase inhibitor therapy. Shaded areas are 95% confidence interval. [Figure adapted and modified from Sanchez A et al., Lancet Oncology, 21(2), 283-293, 2020].
Expanding biomarker efforts which may capture the inflammatory changes associated with obesity also is an area of active investigation. Changes in the neutrophil-to-lymphocyte ratio (NLR) has shown to be a marker for early response to immunotherapy,37 and likely needs to be interpreted in the context of NLR changes which may occur with obesity. Other circulating markers which relate to underlying physiologic changes may also be more apt for this job. Low expression of miR-204-5p, a microRNA associated with obesity, was significantly associated with higher disease recurrence and may enhance risk-based prognostic models.38 Serum adiponectin has been shown to inversely correlate with BMI in RCC patients, yet tissue expression was not associated with disease aggressiveness or survival.39 In addition to leptin, it has been postulated that sex hormones may play a role in modulating ICI therapy, as trends were seen in the melanoma series only in male patients.18 Sun and colleagues raise further questions as they preliminarily showed RCC disease risk differences between pre-menopausal and post-menopausal women.40 Lastly, with the growing understanding of the relationship between the gut microbiome and obesity gut dysbiosis-related changes with ICI therapy,41 linking these findings may help inform other biomarker discovery efforts in the future.
Looking forward, augmenting cancer immunotherapy with obesity-related strategies to improve immune cell fitness or by inhibiting obesity-related immune dysregulation remains a high priority. Several drugs that are commonly used in this patient population have previously been shown to have beneficial effects when combined with ICI therapy. Fenofibrate, a common lipid-lowering drug, was found to synergize with ICI blockade in melanoma models via activation of the PPARα pathway.42 Metformin, a diabetes drug commonly implicated in cancer risk reduction, was also shown to synergize with ICI therapy via increased tumor oxidative phosphorylation and reduced tumor hypoxia.43 Given the upregulation of pseudohypoxia-related pathways in VHL-driven clear cell RCC, understanding this combinatorial strategy in a RCC tumor model may uncover unique insights. As leptin partly exerts activity through activation of Jak/STAT pathways, many have proposed downstream modulation of these specific pathways with currently approved in combination with ICI therapy. As immune related changes have been shown after even lifestyle adjustments like dietary modifications, exercise and psychoeducation,44 understanding the contribution of these interventions within this population may ultimately improve ICI outcomes and the relative overall health of our patients.
Extrapolating these paradigms outside of clear cell RCC histology may uncover new insights and applications for rare, non-clear cell RCC tumors where there remains a high unmet need. Data from the Kaiser Permanente network highlighted that the obesity paradox was associated with clear cell and chromophobe tumors but not papillary RCC, and this was confirmed in their meta-analysis showing a relative risk of 1.8 and 2.2 for clear cell and chromophobe histologies, respectively, and 1.2 for papillary tumors.11 A large case-control series in the United States and Europe also confirmed this same subtype-specific trend.13 As chromophobe RCC tumors are notoriously resistant to ICI therapy, 45 evaluating whether immune microenvironment shifts which may be related to the obesity paradox are paralleled to the changes seen in clear cell RCC may help refine immune-based strategies in this specific tumor subtype.
FINAL REMARKS
The field has rapidly evolved from the first sighting of obesity-related trends to a more mechanistic grasp on the links between obesity, immunity, and cancer biology. As new surrogates and biomarkers are developed that interrogate individual tumors within their surrounding tissue borders and microenvironment, combinatorial strategies which leverage this metabolic data to enhance immune cell fitness and efficacy remains on the horizon. Further, with advances in our understanding of the physiologic impact of obesity itself, we will soon be better positioned to improve the health and lives of our patients. With accelerating rates of obesity worldwide and the rising health and economic tolls, now, more than ever, it is critical to undertake further exploration of mechanism-based strategies to improve precision-based care in this patient population.
KEYWORDS: • Renal Cell Carcinoma • Obesity Paradox • ImmunotherapyREFERENCES
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med. 2019;381(25):2440-2450.
- Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794-798.
- Hong Y-R, Huo J, Desai R, Cardel M, Deshmukh AA. Excess Costs and Economic Burden of Obesity-Related Cancers in the United States. Value in Health. 2019;22(12):1378-1386.
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. The Lancet. 2008;371(9612):569-578.
- Wang F, Xu Y. Body mass index and risk of renal cell cancer: a dose-response meta-analysis of published cohort studies. Int J Cancer. 2014;135(7):1673-1686.
- Adams KF, Leitzmann MF, Albanes D, et al. Body size and renal cell cancer incidence in a large US cohort study. American journal of epidemiology. 2008;168(3):268-277.
- Johansson M, Carreras-Torres R, Scelo G, et al. The influence of obesity-related factors in the etiology of RCC-A mendelian randomization study. PLoS medicine. 2019;16(1):e1002724.
- Choi Y, Park B, Jeong BC, et al. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer. 2013;132(3):625- 634.
- Donin NM, Pantuck A, Klöpfer P, et al. Body Mass Index and Survival in a Prospective Randomized Trial of Localized High-Risk RCC. Cancer Epidemiology Biomarkers Prevention. 2016;25(9):1326.
- Hötker AM, Karlo CA, Di Paolo PL, et al. Renal cell carcinoma: Associations between tumor imaging features and epidemiological risk factors. European Journal of Radiology. 2020;129:109096.
- Callahan CL, Hofmann JN, Corley DA, et al. Obesity and renal cell carcinoma risk by histologic subtype: A nested case-control study and meta-analysis. Cancer epidemiology. 2018;56:31-37.
- Hakimi AA, Furberg H, Zabor EC, et al. An epidemiologic and genomic investigation into the obesity paradox in RCC Journal of the National Cancer Institute. 2013;105(24):1862-1870.
- Purdue MP, Moore LE, Merino MJ, et al. An investigation of risk factors for renal cell carcinoma by histologic subtype in two case-control studies. 2013;132(11):2640-2647.
- Steffens S, Ringe KI, Schroeer K, et al. Does overweight influence the prognosis of renal cell carcinoma? Results of a multicenter study. 2013;20(6):585-592.
- Sunela KL, Kataja MJ, Kellokumpu- Lehtinen P-LI. Influence of BMI and Smoking on the Long-Term Survival of Patients With RCC. Clinical Genitourinary Cancer. 2013;11(4):458-464.
- Martini DJ, Liu Y, Shabto JM, et al. Novel Risk Scoring System for Patients with mRCC Treated with Immune Checkpoint Inhibitors. The oncologist. 2020;25(3):e484-e491.
- Albiges L, Hakimi AA, Xie W, et al. BMI and mRCC: Clinical and Biological Correlations. J Clin Oncol. 2016;34(30):3655- 3663.
- McQuade JL, Daniel CR, Hess KR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19(3):310-322.
- Labadie BW, Liu P, Bao R, et al. BMI, irAE, and gene expression signatures associate with resistance to immune-checkpoint inhibition and outcomes in RCC. Journal of translational medicine. 2019;17(1):386.
- Lalani A-KA, Xie W, Flippot R, et al. Impact of body mass index (BMI) on treatment outcomes to immune checkpoint blockade (ICB) in mRCC. Journal of Clinical Oncology. 2019;37(7_suppl):566-566.
- Cortellini A, Bersanelli M, Buti S, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/ PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. Journal for ImmunoTherapy of Cancer. 2019;7(1):57.
- Elias R, Yan F, Singla N, et al. Immune-related adverse events are associated with improved outcomes in ICI-treated RCC patients. Journal of Clinical Oncology. 2019;37(7_suppl):645-645.
- Guzman-Prado Y, Ben Shimol J, Samson O. Body mass index and immune-related adverse events in patients on immune checkpoint inhibitor therapies: a systematic review and meta-analysis. Cancer Immunology, Immunotherapy. 2020.
- Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus Axitinib versus Sunitinib for aRCC. NEJM. 2019.
- Finlay DK, Rosenzweig E, Sinclair LV, et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. Journal of Experimental Medicine. 2012;209(13):2441- 2453.
- Sanchez A, Furberg H, Kuo F, et al. Transcriptomic signatures related to obesity paradox in patients with ccRCC: a cohort study. Lancet Oncol. 2020;21(2):283-293.
- Haake SM, Brooks SA, Welsh E, et al. Patients with ClearCode34-identified molecular subtypes of ccRCC represent unique populations with distinct comorbidities. Urol Oncol. 2016;34(3):122.e121-127.
- James BR, Tomanek-Chalkley A, Askeland EJ, Kucaba T, Griffith TS, Norian LA. Diet-induced obesity alters dendritic cell function in the presence and absence of tumor growth. J Immunol. 2012;189(3):1311-1321.
- Hale M, Itani F, Buchta CM, Wald G, Bing M, Norian LA. Obesity triggers enhanced MDSC accumulation in murine renal tumors via elevated local production of CCL2. PLoS One. 2015;10(3):e0118784.
- Gibson JT, Norris KE, Wald G, et al. Obesity induces limited changes to systemic and local immune profiles in treatment-naive human ccRCC. PloS one. 2020;15(5):e0233795-e0233795.
- Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nature Medicine. 2019;25(1):141-151.
- Horiguchi A, Sumitomo M, Asakuma J, et al. Increased Serum Leptin Levels and Over Expression of Leptin Receptors are Associated With the Invasion and Progression of Renal Cell Carcinoma. 2006;176(4):1631-1635.
- Cortellini A, Bozzetti F, Palumbo P, et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: a multicenter real-life study. Scientific Reports. 2020;10(1):1456.
- Auclin E, Bourillon C, De Maio E, et al. Prediction of Everolimus Toxicity and Prognostic Value of Skeletal Muscle Index in Patients With mRCC. Clinical Genitourinary Cancer. 2017;15(3):350-355.
- Mano R, Hakimi AA, Zabor EC, et al. Association between visceral and subcutaneous adiposity and clinicopathological outcomes in non-metastatic ccRCC. Can Urol Assoc J. 2014;8(9-10):E675-E680.
- Lalani AA, Xie W, Martini DJ, et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for mRCC. J Immunother Cancer. 2018;6(1):5.
- Shu X, Hildebrandt MA, Gu J, et al. MicroRNA profiling in clear cell renal cell carcinoma tissues potentially links tumorigenesis and recurrence with obesity. British Journal of Cancer. 2017;116(1):77-84.
- Ito R, Narita S, Huang M, et al. The impact of obesity and adiponectin signaling in patients with renal cell carcinoma: A potential mechanism for the “obesity paradox”. PLoS One. 2017;12(2):e0171615.
- Sun L, Chao F, Luo B, et al. Impact of Estrogen on the Relationship Between Obesity and RCC Risk in Women. EBioMedicine. 2018;34:108-112.
- Derosa L, Routy B, Fidelle M, et al. Gut Bacteria Composition Drives Primary Resistance to Cancer Immunotherapy in RCC Patients. European Urology. 2020;78(2):195- 206.
- Zhang Y, Kurupati R, Liu L, et al. Enhancing CD8(+) T Cell Fatty Acid Catabolism within a Metabolically Challenging Tumor Microenvironment Increases the Efficacy of Melanoma Immunotherapy. Cancer Cell. 2017;32(3):377-391.e379.
- Scharping NE, Menk AV, Whetstone RD, Zeng X, Delgoffe GM. Efficacy of PD-1 Blockade Is Potentiated by Metformin-Induced Reduction of Tumor Hypoxia. Cancer Immunol Res. 2017;5(1):9-16.
- van der Zalm IJB, van der Valk ES, Wester VL, et al. Obesity-associated T-cell and macrophage activation improve partly after a lifestyle intervention. International Journal of Obesity. 2020.
- Ged Y, Chen Y-B, Knezevic A, et al. Metastatic Chromophobe Renal Cell Carcinoma: Presence or Absence of Sarcomatoid Differentiation Determines Clinical Course and Treatment Outcomes. Clinical Genitourinary Cancer. 2019;17(3):e678-e688.