NOW ENROLLING
Bristol Myers Squibb (BMS) is currently recruiting candidates for CheckMate 914 study, to evaluate the potential role of immuno-oncology agents nivolumab and ipilimumab for early-stage, high-risk renal cell carcinoma (RCC)
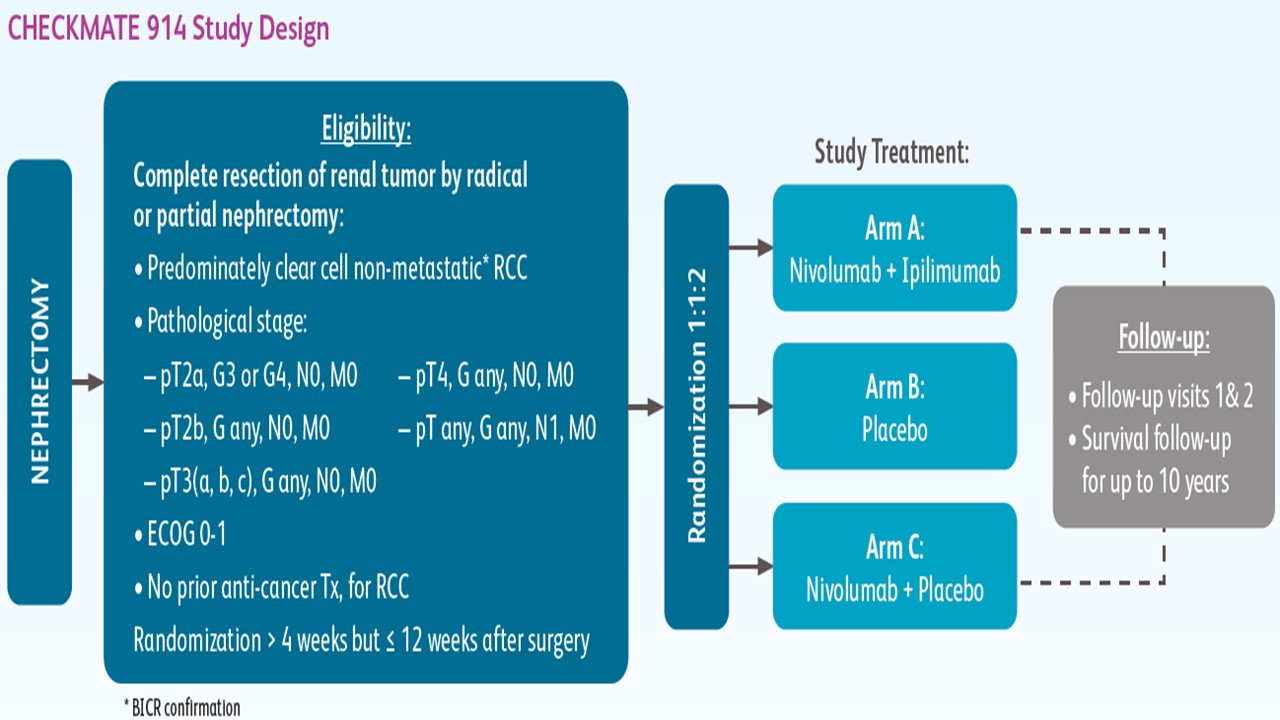
CheckMate 914 is a randomized, phase 3 double blind clinical trial evaluating adjuvant nivolumab alone or in combination with ipilimumab vs Placebo in patients who underwent radical or partial nephrectomy and who are at high risk of relapse.
As registered with ClinicalTrials.gov (NCT03138512), the CheckMate 914 trial is comprised of patients who underwent radical or partial nephrectomy with negative surgical margins at least four weeks and less than 12 weeks prior to randomization with a targeted accrual of 1,600 patients. All treatments are given for 24 weeks or until disease recurrence, unacceptable toxicity, or withdrawal of consent. The purpose of this study is to determine whether nivolmab alone or the combination of nivolumab and ipilimumab versus placebo, is safe and effective for delaying or preventing recurrence of cancer in patients who have experienced partial or entire removal of a kidney. The primary outcome is disease-free survival according to blinded independent central review. Key secondary outcomes include overall survival.
To find out if your patients are eligible for this trial, check out below link:
![]()
ELIGIBILITY:
Inclusion Criteria:
Exclusion Criteria:
* Other protocol defined inclusion/exclusion criteria apply
The newly developed recommendations by NCI and FDA allows increasing flexibility for trial investigators to reduce COVID-19 exposure or offers alternative care settings to improve the overall trial process during the COVID-19 pandemic.