ASCO21 Sessions:
RECOMMENDED ABSTRACTS in Renal Cancer

These recommended abstracts have been selected by Robert A. Figlin, MD, Editor-in- Chief of the Kidney Cancer Journal. The chosen abstracts provided here highlight some of the most important trends in ongoing trials and reflect the foremost research and strategies from latest clinical trials that impact the current standard of care in renal cancer.



ABSTRACT 4500:
Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): Results from 42-month follow-up of KEYNOTE-426
Brian I. Rini, Elizabeth R. Plimack, Viktor Stus, Tom Waddell, Rustem Gafanov, Frédéric Pouliot, Dmitry Nosov, Bohuslav Melichar, Denis Soulieres, Delphine Borchiellini, Ihor O. Vynnychenko, Raymond S. McDermott, Sergio Jobim Azevedo, Satoshi Tamada, Anna Kryzhanivska, Chenxiang Li, Joseph E. Burgents, L. Rhoda Molife, Jens Bedke, Thomas Powles; Vanderbilt-Ingram Cancer Center, Nashville, TN; Fox Chase Cancer Center, Philadelphia, PA; Dnipropetrovsk Medical Academy of Ministry of Health of Ukraine, Dnipro, Ukraine; The Christie NHS Foundation Trust, Manchester, United Kingdom; Russian Scientific Center of Roentgenoradiology, Moscow, Russian Federation; CHU of Québec and Laval University, Québec City, ON, Canada; Central Clinical Hospital With Outpatient Clinic, Moscow, Russian Federation; Palacky University Medical School and Teaching Hospital, Olomouc, Czech Republic; Centre Hospitalier de l'Université de Montréal, Montréal, QC, Canada; Centre Antoine Lacassagne, Université Côte d’Azur, Nice, France; Sumy State University, Sumy Regional Oncology Center, Sumy, Ukraine; Adelaide and Meath Hospital and University College Dublin, Dublin, Ireland; Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil; Osaka City University Hospital, Osaka, Japan; Ivano-Frankivsk National Medical University, Ivano-Frankivsk, Ukraine; Merck & Co., Inc., Kenilworth, NJ; MSD UK, London, United Kingdom; Eberhard Karls Universität Tübingen, Tübingen, Germany; Barts Health and the Royal Free NHS Trusts, Barts Cancer Institute, Queen Mary University of London, London, United Kingdom
Background: In the first interim analysis of the randomized, multicenter, open-label, phase 3 KEYNOTE-426 study (NCT02853331), treatment with pembro + axi significantly improved OS, PFS, and ORR vs sunitinib monotherapy in treatment-naive advanced ccRCC. Extended follow-up (median, 30.6 mo) continued to demonstrate the superior efficacy of pembro + axi vs sunitinib monotherapy in this patient population. Here, we present the results of the prespecified final analysis with 42.8-mo median follow-up.
Methods: Treatment-naive patients (pts) with advanced ccRCC, KPS ≥70%, and measurable disease (RECIST v1.1) were randomly assigned 1:1 to receive pembro 200 mg IV Q3W for up to 35 doses + axi 5 mg orally BID or sunitinib 50 mg orally QD on a 4-wk on/2-wk off schedule until progression, intolerable toxicity, or withdrawal. Randomization was stratified by IMDC risk (favorable vs intermediate vs poor) and geographic region (North America vs Western Europe vs Rest of World). Dual primary endpoints were OS and PFS. Secondary endpoints were ORR, DOR, and safety. The protocol-specified final analysis was based on a target of 404 OS events. No formal hypothesis testing was performed because all efficacy endpoints were met previously at the first interim analysis; nominal P values are reported.
Results: Overall, 861 pts were randomly assigned to receive pembro + axi (n=432) or sunitinib (n=429). Median duration of follow-up, defined as time from randomization to the database cutoff date, was 42.8 mo (range, 35.6-50.6). At data cutoff, 418 pts had died: 193 (44.7%) of 432 pts in the pembro + axi arm vs 225 (52.4%) of 429 pts in the sunitinib arm. Compared with sunitinib, pembro + axi improved OS (median: 45.7 vs 40.1 mo; HR, 0.73 [95% CI, 0.60-0.88]; P<0.001) and PFS (median: 15.7 vs 11.1 mo; HR, 0.68 [95% CI, 0.58-0.80]; P<0.0001). The 42-mo OS rate was 57.5% with pembro + axi vs 48.5% with sunitinib; the 42-mo PFS rate was 25.1% with pembro + axi vs 10.6% with sunitinib. For pembro + axi vs sunitinib, ORR was 60.4% vs 39.6% (P<0.0001); CR rate was 10.0% vs 3.5%; median DOR was 23.6 mo (range 1.4+ to 43.4+) vs 15.3 mo (range, 2.3-42.8+). Subsequent anticancer therapy was administered to 47.2% of pts in pembro + axi arm vs 65.5% of pts in sunitinib arm. Although a similar proportion of pts in each arm received VEGF/VEGFR inhibitors, only 10.2% of pts in the pembro + axi arm received subsequent treatment with a PD-1/L1 inhibitor compared to 48.7% of pts in the sunitinib arm. No new safety signals were observed. Conclusions: With a median follow-up of 42.8 mo, this is the longest follow-up of an anti-PD–1/L1 immunotherapy combined with a VEGF/VEGFR inhibitor for first-line RCC. These results show that pembro + axi continues to demonstrate superior efficacy over sunitinib with respect to OS, PFS, and ORR, with no new safety signals.
Clinical trial information: NCT02853331
Research Funding: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA


ABSTRACT 4501 :
CANTATA: Primary analysis of a global, randomized, placebo (Pbo)-controlled, double-blind trial of telaglenastat (CB-839) + cabozantinib versus Pbo + cabozantinib in advanced/metastatic renal cell carcinoma (mRCC) patients (pts) who progressed on immune checkpoint inhibitor (ICI) or anti-angiogenic therapies.
Nizar M. Tannir, Neeraj Agarwal, Camillo Porta, Nicola Jane Lawrence, Robert J. Motzer, Richard J. Lee, Rohit K. Jain, Nancy B. Davis, Leonard Joseph Appleman, Oscar B. Goodman, Walter Michael Stadler, Sunil G. Gandhi, Daniel M. Geynisman, Roberto Iacovelli, Begona Mellado, Robert A. Figlin, Thomas Powles, Lalith V Akella, Keith W. Orford, Bernard Escudier; The University of Texas MD Anderson Cancer Center, Houston, TX; Huntsman Cancer Institute, University of Utah, Salt Lake City, UT; University of Bari 'A. Moro' and Policlinico Consorziale di Bari, Bari, Italy; Auckland City Hospital, Auckland, New Zealand; Memorial Sloan Kettering Cancer Center, New York, NY; Massachusetts General Hospital, Boston, MA; H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL; Vanderbilt-Ingram Cancer Center, Nashville, TN; University of Pittsburgh Medical Center, Pittsburgh, PA; Comprehensive Cancer Centers of Nevada, Las Vegas, NV; The University of Chicago, Chicago, IL; Florida Cancer Specialists, Lecanto, FL; Fox Chase Cancer Center, Department of Hematology and Oncology, Philadelphia, PA; Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Hospital Clínic, Provincial de Barcelona, Barcelona, Spain; Cedars-Sinai Medical Center, Samuel Oschin Comprehensive Cancer Institute, Los Angeles, CA; St Bartholomew's Hospital, Barts Health NHS Trust, London, United Kingdom; Calithera Biosciences, Inc., South San Francisco, CA; Gustave Roussy, Villejuif, France
Background:Dysregulated metabolism is a hallmark of RCC, driven by overexpression of glutaminase (GLS), a key enzyme of glutamine metabolism. Telaglenastat (Tela) is an investigational, first-in-class, selective, oral GLS inhibitor that blocks glutamine utilization and critical downstream pathways. Preclinically, Tela synergized w/ cabozantinib (Cabo), a VEGFR2/MET/AXL inhibitor, against RCC tumors. In a Ph 1 study cohort, Tela+Cabo showed encouraging safety/efficacy as 2L+ therapy for mRCC. This trial compared Tela+Cabo vs Pbo+Cabo in previously treated pts w/ clear-cell mRCC (NCT03428217).
Methods: Eligible pts had 1-2 prior lines of systemic therapy for mRCC, including ≥1 anti-angiogenic therapy or nivolumab + ipilimumab (nivo/ipi), KPS ≥70%, measurable disease (RECIST 1.1), no prior Cabo or other MET inhibitor. Pts were randomized 1:1 to receive Cabo (60 mg PO QD) with either Tela (800 mg PO BID) or Pbo, until disease progression/unacceptable toxicity, and were stratified by prior PD-(L)1 inhibitor therapy (Y/N) and IMDC prognostic risk group. Primary endpoint was progression-free survival (PFS; RECIST 1.1) by blinded independent radiology review. The study was designed to detect a PFS hazard ratio (HR) of 0.69 w/ alpha 0.05 and 85% power. Data cutoff date: August 31, 2020.
Results: 444 pts were randomized (221 Tela+Cabo; 223 Pbo+Cabo). Baseline characteristics were balanced between arms. Median follow-up was 11.7 mo; 276 pts received prior ICI, including 128 w/ prior nivo/ipi. Median PFS (mPFS) was 9.2 mo for Tela+Cabo vs 9.3 mo for Pbo+Cabo (HR = 0.94; 95% CI: 0.74, 1.21; stratified log-rank P= 0.65) with overall response rates (ORR; confirmed) of 31% with Tela+Cabo vs 28% Pbo+Cabo, respectively. Overall survival was not mature at data cutoff. In a prespecified subgroup analysis in pts w/ prior ICI, mPFS was numerically longer w/ Tela+Cabo than Pbo+Cabo (11.1 vs 9.2 mo, respectively; unstratified HR = 0.77; 95% CI: 0.56, 1.06). In the Pbo+Cabo arm, mPFS was 9.2 mo for pts w/ prior ICI exposure and 9.5 mo for pts without, and ORR was 32% and 20%, respectively; if ICI included nivo/ipi, ORR was 37%. Rates of adverse events (AEs) were similar between arms.Grade 3-4 AEs occurred in 71% of Tela+Cabo pts and 79% of Pbo+Cabo pts and included hypertension (17% vs 18%) and diarrhea (15% vs 13%). Cabo was discontinued due to AEs in 10% of Tela+Cabo pts and 15% of Pbo+Cabo pts.
Conclusions: The addition of Tela did not improve the efficacy of Cabo in mRCC in this study. Tela+Cabo was well tolerated with AEs consistent with known risks of both agents. The study provides valuable insight on efficacy outcomes of a contemporary population of pts w/ mRCC who receive Cabo in the 2/3L setting.
Clinical trial information: NCT03428217
Research Funding: Calithera Biosciences, Inc


LATE BREAKTHROUGH ABSTRACT LBA 5 :
Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for patients with renal cell carcinoma: Randomized, double-blind, phase III KEYNOTE-564 study.
Toni K. Choueiri, Piotr Tomczak, Se Hoon Park, Balaji Venugopal, Tom Ferguson, Yen-Hwa Chang, Jaroslav Hajek, Stefan N. Symeonides, Jae-Lyun Lee, Naveed Sarwar, Antoine Thiery-Vuillemin, Marine Gross-Goupil, Mauricio Mahave, Naomi B. Haas, Piotr Sawrycki, Eric (Pingye) Zhang, Jaqueline Willemann Rogerio, Kentaro Imai, David I. Quinn, Thomas Powles; Dana-Farber Cancer Institute, Boston, MA; Szpital Kliniczny Przemienienia Pańskiego UM, Poznan, Poland; Division of Hematology-Oncology, Samsung Medical Center, Department of Medicine, Seoul, South Korea; University of Glasgow, Glasgow, United Kingdom; Royal Perth Hospital, Perth, Australia; Department of Urology, Taipei Veterans General Hospital, Taipei, Taiwan; Fakultni Nemocnice Ostrava, Ostrava, Czech Republic; Edinburgh Cancer Research Centre, University of Edinburgh, Edinburgh, United Kingdom; Asan Medical Center and University of Ulsan College of Medicine, Seoul, South Korea; Imperial College Healthcare NHS Trust, London, United Kingdom; University Hospital Jean Minjoz, Besançon, France; Centre Hospitalier Universitaire de Bordeaux-Hôpital Saint-André, Bordeaux, France; Lopez Perez Foundation, Santiago, Chile; Abramson Cancer Center, University of Pennsylvania (ECOG-ACRIN), Philadelphia, PA; Wojewodzki Szpital Zespolony im. L. Rydygiera w Toruniu, Torun, Poland; Merck & Co., Inc., Kenilworth, NJ; USC Norris Cancer Hospital, Los Angeles, CA; Barts Cancer Institute, Cancer Research UK Experimental Cancer Medicine Centre, Queen Mary University of London, Royal Free National Health Service Trust,, London, United Kingdom
Background:Relapse after surgery for high-risk clear cell RCC (ccRCC) is associated with shortened life expectancy. Effective perioperative therapy to reduce this risk remains an unmet need. Adjuvant immune therapy is an attractive potential strategy for these pts. We conducted the KEYNOTE-564 trial to evaluate pembro vs placebo as adjuvant therapy for pts with RCC.
Methods: KEYNOTE-564 is a phase III multicenter trial of pembro vs placebo in pts with histologically confirmed ccRCC, with intermediate-high risk (pT2, Gr 4 or sarcomatoid, N0 M0; or pT3, any Gr, N0 M0), high risk (pT4, any Gr, N0 M0; or pT any stage, any Gr, N+ M0), or M1 NED (no evidence of disease after primary tumor + soft tissue metastases completely resected ≤1 year from nephrectomy) (Leibovich et al, 2003; Fuhrman et al, 1982). Pts had undergone surgery ≤12 wks prior to randomization; had no prior systemic therapy; had ECOG PS 0 or 1. Study treatment was given for up to 17 cycles (≈1 yr). The primary endpoint was disease-free survival (DFS) per investigator assessment in all randomized pts (ITT population). Overall survival (OS) was a key secondary endpoint. Safety/tolerability were secondary endpoints, assessed in all treated pts.
Results: Between Jun 30, 2017 and Sept 20, 2019, 994 pts were randomized 1:1 to pembro (n=496) or placebo (n=498). As of data cutoff date of Dec 14, 2020, median (range) follow-up, defined as time from randomization to data cutoff, was 24.1 (14.9−41.5) mo. No pts remain on study treatment. Baseline characteristics were generally balanced between arms. At first prespecified interim analysis, the primary endpoint of DFS was met (median not reached [NR] for both arms, HR 0.68, 95% CI 0.53−0.87; P=0.0010 [one-sided]). The estimated DFS rate at 24 mo was 77.3% with pembro vs 68.1% with placebo. Overall, DFS benefit was consistent across subgroups. A total of 51 OS events were observed (18 in the pembro arm, 33 in the placebo arm). Median OS was NR for both arms (HR 0.54, 95% CI 0.30−0.96; P=0.0164 [one-sided]); the p-value did not cross the statistical hypothesis testing boundary. The estimated OS rate at 24 mo was 96.6% with pembro vs 93.5% with placebo. 470 pts (96.3%) and 452 pts (91.1%) experienced ≥1 all-cause adverse events (AEs) with pembro vs placebo, respectively. Grade 3-5 all-cause AEs occurred in 158 pts (32.4%) with pembro and 88 pts (17.7%) with placebo. No deaths related to pembro occurred.
Conclusions: Pembro demonstrated a statistically significant and clinically meaningful improvement in DFS vs placebo in pts with intermediate-high, high risk or M1 NED RCC. Additional follow-up is planned for the key secondary endpoint of OS. KEYNOTE-564 is the first positive phase III study with a checkpoint inhibitor in adjuvant RCC, and these results support pembro as a potential new standard of care for pts with RCC in the adjuvant setting.
Clinical trial information: NCT03142334
Research Funding: Eisai Inc., Woodcliff Lake, NJ, USA, and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA




ABSTRACT 4502 :

Health-related quality-of-life (HRQoL) analysis from the phase 3 CLEAR trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) or everolimus (EVE) versus sunitinib (SUN) for patients (pts) with advanced renal cell carcinoma (aRCC).
Robert J. Motzer, Camillo Porta, Boris Alekseev, Sun Young Rha, Toni K. Choueiri, Maria Jose Mendez-Vidal, Sung-Hoo Hong, Anil Kapoor, Jeffrey C. Goh, Masatoshi Eto, Jinyi Wang, Janice Pan, Alemseged Ayele Asfaw, Cixin Steven He, Kalgi Mody, David Cella; Memorial Sloan Kettering Cancer Center, New York, NY; San Matteo University Hospital Foundation, Pavia, Italy; P.A. Herzen Moscow Oncological Research Institute, Moscow, Russian Federation; Yonsei Cancer Center, Yonsei University Health System, Seoul, South Korea; Dana-Farber Cancer Institute, Boston, MA; Maimonides Institute for Biomedical Research of Cordoba (IMIBIC), Hospital Universitario Reina Sofía, Córdoba, Spain; Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, South Korea; McMaster University Hamilton, Hamilton, ON, Canada; ICON Research, South Brisbane & University of Queensland, St Lucia, QLD, Australia; Kyushu University, Fukuoka, Japan; RTI Health Solutions, Research Triangle Park, NC; Eisai Inc., Woodcliff Lake, NJ; Merck & Co., Inc., Kenilworth, NJ; Northwestern University, Chicago, IL
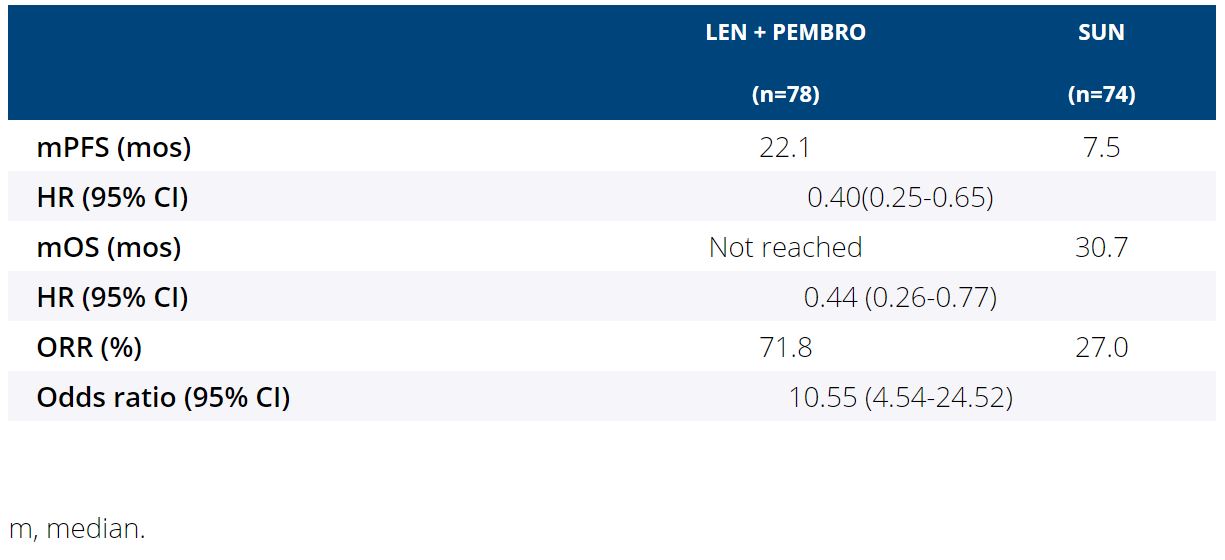
Background:LEN + PEMBRO improved PFS, OS and ORR vs SUN in the first-line treatment of pts with aRCC; LEN + EVE improved PFS and ORR vs SUN (Motzer R et al. NEJM. 2021). We report results of a secondary objective of the CLEAR trial comparing the impact of LEN + PEMBRO or EVE vs SUN, on HRQoL.
Methods: Pts (N=1069) were randomized (1:1:1) to receive LEN 20 mg PO QD + PEMBRO 200 mg IV Q3W; LEN 18 mg + EVE 5 mg PO QD; or SUN 50 mg PO QD (4 wks on/2 wks off). HRQoL was assessed per FKSI-DRS, EORTC QLQ-C30, and EuroQoL EQ-5D-3L, at baseline, on day 1 of subsequent 3 wk cycles starting with cycle 2, and at the off-treatment visit. HRQoL analyses (unless otherwise noted) were based on data from randomized pts with any HRQoL data who received ≥1 dose of study treatment. No adjustments for multiple testing or estimation were used; P-values and CIs are nominal and descriptive.
Results: For comparisons of LEN + PEMBRO vs SUN, overall changes from baseline at mean follow-up (wk 46) favored LEN + PEMBRO with significant differences between treatments for physical functioning (least squares mean difference [LS MD] [95% CI]: 3.0 [0.5, 5.5]) and fatigue (−2.8 [−5.5, −0.1]), dyspnea (−2.8 [−5.3, −0.3]), and constipation (−2.2 [−4.2, −0.2]). LS MD of the FKSI-DRS total score was 0.2 (−0.4, 0.7). For comparisons of LEN + EVE vs SUN, overall changes from baseline at wk 46 favored SUN with significant differences in overall HRQoL (−2.8 [−5.1, −0.5] assessed by the EORTC QLQ-C30 GHS/QoL scale) and pain (2.8 [0.1, 5.5]), appetite loss (4.2 [1.3, 7.1]), and diarrhea (5.3 [2.6, 7.9]). LS MD of the FKSI-DRS total score was −0.4 (−1.0, 0.2). 14 of 18 scales for both LEN + PEMBRO and LEN + EVE vs SUN had no significant differences in LS MD comparisons. The LEN + PEMBRO arm is favored over SUN for the median time to first deterioration (TTD) for physical functioning, dyspnea, appetite loss and EQ-5D VAS (Table). 15 of 19 scales for both LEN + PEMBRO and LEN + EVE vs SUN had no significant differences in TTD comparisons.
Conclusions: Compared with SUN, pts in LEN + PEMBRO group had similar or better symptoms and HRQoL.

Clinical trial information: NCT02811861
Research Funding: Eisai Inc., Woodcliff Lake, NJ, USA, and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA


ABSTRACT 4509 :
Nivolumab plus cabozantinib in patients with non-clear cell renal cell carcinoma: Results of a phase 2 trial.
Chung-Han Lee, Martin H Voss, Maria Isabel Carlo, Ying-Bei Chen, Eduard Reznik, Andrea Knezevic, Robert A Lefkowitz, Natalie Shapnik, Diana Tassone, Chloe Dadoun, Neil J. Shah, Colette Ngozi Owens, Deaglan Joseph McHugh, David Henry Aggen, Andrew Leonard Laccetti, Ritesh Kotecha, Darren R. Feldman, Robert J. Motzer; Memorial Sloan Kettering Cancer Center, New York, NY; Columbia University Medical Center, New York, NY; MD Anderson Cancer Center, Houston, TX
Background:Cabozantinib plus nivolumab (CaboNivo) improved objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) over sunitinib in a phase 3 trial for metastatic clear cell renal cell carcinoma (RCC). (Choueiri, abstract 6960, ESMO 2020) We report the results of a phase 2 trial of CaboNivo in patients (pts) with non-clear cell RCC.
Methods: Pts had advanced non-clear cell RCC, 0 or 1 prior systemic therapies excluding prior immune checkpoint inhibitors, and measurable disease by RECIST. Cabo 40 mg/day plus Nivo 240 mg every 2 weeks or 480 mg every 4 weeks was given across two cohorts. Cohort 1: papillary, unclassified, or translocation associated RCC; Cohort 2: chromophobe RCC. The primary endpoint was ORR by RECIST; secondary endpoints included PFS, OS, and safety. Cohort 1 was a single stage design that met its primary endpoint and was expanded to produce more precise estimates of ORR. Cohort 2 was a Simon two-stage design that closed early for lack of efficacy. Correlative analyses by next generation sequencing were performed and to be presented.
Results: A total of 40 pts were treated in Cohort 1, and 7 pts were treated in Cohort 2 (data cutoff: Jan 20, 2021). Median follow up time was 13.1 months (range 2.2 – 28.6). In Cohort 1, 26 (65%) pts were previously untreated, and 14 (35%) pts had 1 prior line: 10 (25%) received prior VEGF-targeted therapy and 8 (20%) received prior mTOR-targeted therapy. ORR for Cohort 1 was 48% (95% CI 31.5–63.9; Table). Median PFS was 12.5 months (95% CI 6.3–16.4) and median OS was 28 months (95% CI 16.3–NE). No responses were seen among 7 patients in Cohort 2 with chromophobe histology (Table). Grade 3/4 treatment emergent adverse events were consistent with that reported in the phase 3 trial; Grade 3/4 AST and ALT were 9% and 15%, respectively. Cabozantinib and nivolumab were discontinued due to toxicity in 17% and 19% of pts, respectively.
Conclusions: CaboNivo had an acceptable safety profile and showed promising efficacy in metastatic non-clear cell RCC pts with papillary, unclassified, or translocation associated histologies whereas activity in patients with chromophobe RCC was limited.

Clinical trial information: NCT03635892
Research Funding: Exelixis, BMS


ABSTRACT 4555 :
Phase 2 study of belzutifan (MK-6482), an oral hypoxia-inducible factor 2α (HIF-2α) inhibitor, for Von Hippel-Lindau (VHL) disease-associated clear cell renal cell carcinoma (ccRCC).
Ramaprasad Srinivasan, Frede Donskov, Othon Iliopoulos, Wendy Kimryn Rathmell, Vivek Narayan, Benjamin L. Maughan, Stephane Oudard, Tobias Else, Jodi K. Maranchie, Sarah Joanne Welsh, Ananya Roy, Yanfang Liu, Rodolfo F. Perini, W. Marston Linehan, Eric Jonasch; Center for Cancer Research, National Cancer Institute, Bethesda, MD; Aarhus University Hospital, Aarhus, Denmark; Massachusetts General Hospital Cancer Center and Harvard Medical School, Boston, MA; Vanderbilt-Ingram Cancer Center, Nashville, TN; University of Pennsylvania, Philadelphia, PA; University of Utah, Salt Lake City, UT; Hôpital Européen Georges Pompidou, Paris, France; University of Michigan, Ann Arbor, MI; University of Pittsburgh, Pittsburgh, PA; Cambridge University Hospitals NHS Foundation Trust, Cambridge, United Kingdom; Merck & Co., Inc., Kenilworth, NJ; The University of Texas MD Anderson Cancer Center, Houston, TX
Background:Inactivation of VHL leads to aberrant stabilization and accumulation of HIF-2α, which drives tumor growth. Patients (pts) with VHL disease are at risk for ccRCC, pancreatic neuroendocrine tumors (pNETs), and hemangioblastomas. Repeated surgeries are often needed to control ccRCC and other VHL disease manifestations. Prior results of this ongoing open-label phase 2 study (NCT03401788) showed activity with belzutifan in VHL disease. Updated results are presented.
Methods: Adults with germline VHL alterations, measurable and localized/nonmetastatic ccRCC, no prior systemic anticancer therapy, and ECOG PS 0 or 1 received belzutifan 120 mg once daily until progression, intolerable toxicity, or decision to withdraw. The primary end point is ORR of VHL-associated ccRCC tumors per RECIST v1.1 by independent review committee (IRC). Secondary end points include DOR, time to response (TTR), PFS, and safety
Results: As of June 1, 2020, 61 pts enrolled. Most pts (82%) had ECOG PS 0, and the median number of prior tumor reduction procedures (eg, partial nephrectomy, craniotomy, radiation therapy) per pt was 5 (range, 0-15). Lesions outside the kidney (non-RCC tumors) evaluable by IRC included pNETs (33%) and CNS hemangioblastomas (82%). Median follow-up was 69 wk (range, 18-105), median duration of treatment was 68 wk (range, 8-105), and 56 pts (92%) remain on therapy. There were 22 confirmed responses (ORR, 36% [95% CI, 24-49]) and 7 (11%) unconfirmed responses (documented at 1 time point, to be confirmed at subsequent time point); all were PRs. In pts with confirmed PR, median DOR was not reached (range, 12+ to 62+ wk), median TTR was 31 wk (range, 12-61), and 56 pts (92%) had some reduction in the sum of all target lesion diameters. PFS rate at 52 wk was 98% (95% CI, 89-100). For non-RCC tumors, ORR was 80% (16/20; 1 CR) in pNETs and 32% (16/50; 1 CR) in CNS hemangioblastomas. Of 16 pts with evaluable retinal hemangioblastomas at baseline, 11 (69%) showed improvement per IRC. In those 16 pts, 29 eyes were monitored for retinal hemangioblastomas: 16 eyes (55%) showed improvement, 12 (41%) were stable, and no evaluation was available for 1 eye (3%). All 61 pts (100%) had at least one AE. The most common all-cause AE was anemia (90%), which is considered an on-target toxicity. Treatment-related AEs (TRAE) were reported by 60 pts (98%), and 8 pts (13%) had a grade 3 TRAE. No pts had grade 4/5 TRAEs. One pt discontinued treatment because of a TRAE (grade 1 dizziness).
Conclusions: Belzutifan demonstrates clinical benefit and has a favorable safety profile in patients with VHL disease–associated ccRCC, pNETs, and hemangioblastomas.
Clinical trial information: NCT03401788
Research Funding: Research Funding: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA


ABSTRACT 4562:
Post hoc analysis of the CLEAR study in advanced renal cell carcinoma (RCC): Effect of subsequent therapy on survival outcomes in the lenvatinib (LEN) + everolimus (EVE) versus sunitinib (SUN) treatment arms.
Thomas E. Hutson, Toni K. Choueiri, Robert J. Motzer, Sun Young Rha, Anna Alyasova, Jaime R. Merchan, Howard Gurney, Avivit Peer, Toshio Takagi, Camillo Porta, Thomas Powles, Viktor Grünwald, Ugo De Giorgi, Ulka N. Vaishampayan, Manuela Schmidinger, Hilary Glen, Karla Rodriguez-Lopez, Dongyuan Xing, Lea Dutta, Masatoshi Eto; Texas Oncology, Dallas, TX; Dana-Farber Cancer Institute, Boston, MA; Memorial Sloan Kettering Cancer Center, New York, NY; Yonsei Cancer Center, Yonsei University Health System, Seoul, South Korea; Prevoljskiy Region Medical Centre, Novgorod, Russian Federation; University of Miami Sylvester Comprehensive Cancer Center, Miami, FL; Macquarie University Hospital, Sydney, NSW, Australia; Rambam Health Care Campus, Haifa, Israel; Tokyo Women's Medical University, Tokyo, Japan; San Matteo University Hospital Foundation, Pavia, Italy; The Royal Free NHS Trust, London, United Kingdom; University Hospital Essen, Essen, Germany; Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy; University of Michigan, Ann Arbor, MI; Medical University of Vienna, Vienna, Austria; Beatson West of Scotland Cancer Center, Glasgow, United Kingdom; Merck & Co., Inc., Kenilworth, NJ; Eisai Inc., Woodcliff Lake, NJ; Kyushu University, Fukuoka, Japan
Background:
The multicenter, open-label, randomized, phase 3 CLEAR study showed that LEN + EVE had a significant PFS benefit (HR 0.65, 95% CI 0.53-0.80, P<0.001) and improved objective response rate (relative risk 1.48, 95% CI 1.26-1.74) vs SUN in the first-line treatment of patients (pts) with advanced RCC. The difference in overall survival (OS) for LEN + EVE vs SUN was not statistically significant (HR 1.15, 95% CI 0.88-1.50) (Motzer R et al. NEJM. 2021). Post hoc subgroup analyses were performed to assess the impact of subsequent therapy on OS.Methods:
Pts in the CLEAR study were randomly assigned (1:1:1) to 1 of 3 treatment arms, including LEN 18 mg + EVE 5 mg once daily (QD) and SUN 50 mg QD (4 weeks on then 2 weeks off). These post hoc analyses examined OS by subsequent systemic anticancer medication in the LEN + EVE and SUN arms. Hazard ratios (HR; LEN + EVE vs SUN) were based on stratified (geographic region and MSKCC prognostic risk groups) Cox proportional hazards model.Results:
Among 1069 pts with advanced RCC randomized in the CLEAR study, 714 pts were randomly assigned to the LEN + EVE and SUN arms (N=357/each). The median duration of survival follow-up was 27 months in the LEN + EVE arm and 26 months in the SUN arm. Given the shorter median duration of study treatment with SUN (7.8 months) vs LEN + EVE (11.0 months), more pts in the SUN arm received subsequent anticancer therapy during survival follow-up (LEN + EVE, n=167; SUN, n=206). Among pts who received subsequent therapy, pts in the LEN + EVE arm had a longer median time from randomization to initiation of subsequent therapy vs those in the SUN arm (8.0 vs 6.6 months, respectively). OS for the overall population, for pts with no subsequent anticancer therapy, and for pts with no subsequent immunotherapy is shown in the table. In the US population subgroup (LEN + EVE, n=62; SUN, n=61) of the CLEAR study, in which a similar number of pts received subsequent systemic anticancer therapies in the LEN + EVE vs SUN arms (62.9% vs 65.6%, respectively), OS was comparable among the 2 arms (HR 0.95, 95% CI 0.51-1.76). Overall, the safety profile was consistent with the known safety profiles of LEN + EVE and SUN. In both arms, most treatment-emergent deaths were due to progressive disease; there were few treatment-related deaths (<1%, per arm) and no clustering of events.Conclusions:
In the CLEAR study, LEN + EVE met the primary endpoint of a significant benefit in PFS vs SUN. The results of these exploratory analyses suggest that subsequent systemic anticancer therapy affected the OS outcome results for LEN + EVE vs SUN in the CLEAR study. Clinical trial information: NCT02811861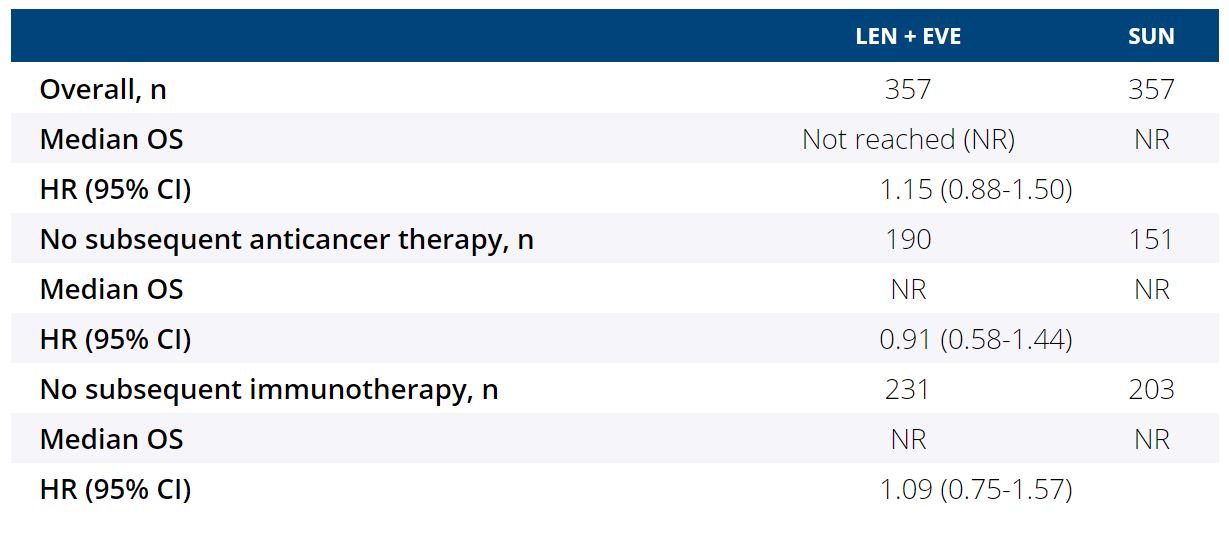
Clinical trial information:NCT02811861
Research Funding:
Eisai Inc., Woodcliff Lake, NJ, USA, and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA


ABSTRACT 4578:

Efficacy outcomes of nivolumab + cabozantinib versus pembrolizumab + axitinib in patients with advanced renal cell carcinoma (aRCC): Matching-adjusted indirect comparison (MAIC).
Bradley Alexander McGregor, Daniel M. Geynisman, Mauricio Burotto, Camillo Porta, Cristina Suarez Rodriguez, Maria Teresa Bourlon, Pedro C. Barata, Shuchi Gulati, Brian Stwalley, Viviana Del Tejo, Ella X. Du, Aozhou Wu, Andi Chin, Keith A. Betts, Stephen Huo, Toni K. Choueiri; Dana-Farber Cancer Institute, Boston, MA; Fox Chase Cancer Center, Department of Hematology and Oncology, Philadelphia, PA; Bradford Hill Clinical Research Center, Santiago, Chile; University of Bari 'A. Moro' and Policlinico Consorziale di Bari, Bari, Italy; Medical Oncology, Vall d´Hebron Institute of Oncology (VHIO), Hospital Universitari Vall d´Hebron, Vall d´Hebron Barcelona Hospital Campus, Barcelona, Spain; Urologic Oncology Clinic, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, DF, Mexico; Tulane Cancer Center, New Orleans, LA; University of Cincinnati Medical Center, Cincinnati, OH; Bristol Myers Squibb, Princeton, NJ; Analysis Group, Inc, Los Angeles, CA; Analysis Group, Inc., Los Angeles, CA; Analysis Group, New York, NY; Dana-Farber Cancer Institute, The Lank Center for Genitourinary Oncology, Boston, MA
Background:Nivolumab in combination with cabozantinib (N+C) has demonstrated significantly improved progression-free survival (PFS), objective response rate (ORR), and overall survival (OS), compared with sunitinib as a first-line (1L) treatment for aRCC in the phase 3 CheckMate (CM) 9ER trial. As there are no head-to-head trials comparing N+C with pembrolizumab in combination with axitinib (P+A), this study compared the efficacy of N+C with P+A as 1L treatment in aRCC.
Methods: An MAIC was conducted using individual patient data on N+C (N = 323) from the CM 9ER trial (median follow-up: 23.5 months) and published data on P+A (N = 432) from the KEYNOTE (KN)-426 trialof P+A (median follow-up: 30.6 months). Individual patients within the CM 9ER trial population were reweighted to match the key patient characteristics published in KN-426 trial, including age, gender, previous nephrectomy, International Metastatic RCC Database Consortium risk score, and sites of metastasis. After weighting, hazards ratios (HR) of PFS, duration of response (DoR), and OS comparing N+C vs. P+A were estimated using weighted Cox proportional hazards models, and ORR was compared using a weighted Wald test. All comparisons were conducted using the corresponding sunitinib arms as an anchor.
Results: After weighting, patient characteristics in the CM 9ER trial were comparable to those in the KN-426 trial. In the weighted population, N+C had a median PFS of 19.3 months (95% CI: 15.2, 22.4) compared to a median PFS of 15.7 months (95% CI: 13.7, 20.6) for P+A. Using sunitinib as an anchor arm, N+C was associated with a 30% reduction in risk of progression or death compared to P+A, (HR: 0.70, 95% CI: 0.53, 0.93; P = 0.015; table). In addition, N+C was associated with numerically, although not statistically, higher improvement in ORR vs sunitinib (difference: 8.4%, 95% CI: -1.7%, 18.4%; P = 0.105) and improved DoR (HR: 0.79; 95% CI: 0.47, 1.31; P = 0.359). Similar OS outcomes were observed for N+C and P+A (HR: 0.99; 95% CI: 0.67, 1.44; P = 0.940).
Conclusions: After adjusting for cross-trial differences, N+C had a more favorable efficacy profile compared to P+A, including statistically significant PFS benefits, numerically improved ORR and DoR, and similar OS.
Research Funding: BMS




Newly Approved Drug Section
ABSTRACT 4567:
Temporal characteristics of treatment-emergent adverse events and dose modifications with tivozanib and sorafenib in the phase 3 TIVO-3 study of relapsed or refractory mRCC.
Sumanta K. Pal, David F. McDermott, Bernard Escudier, Thomas E. Hutson, Camillo Porta, Elena Verzoni, Michael B. Atkins, Michael N. Needle, Brian I. Rini; Department of Medical Oncology & Therapeutics, City of Hope Comprehensive Cancer Center, Duarte, CA; Beth Israel Deaconess Medical Center, Dana-Farber/Harvard Cancer Center, Boston, MA; Gustave Roussy, Villejuif, France; Texas A&M College of Medicine, Bryan, TX; University of Bari 'A. Moro' and Policlinico Consorziale di Bari, Bari, Italy; Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy; Georgetown Lombardi Comprehensive Cancer Center, Washington, DC; Aveo Oncology, Boston, MA; Vanderbilt-Ingram Cancer Center, Nashville, TN
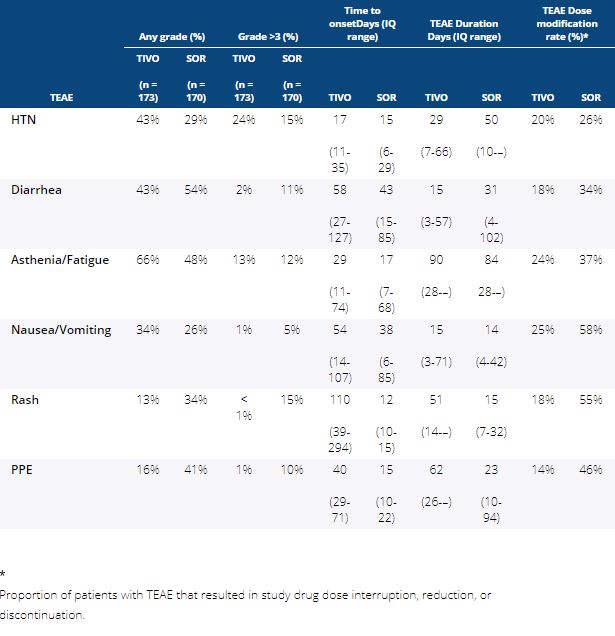
Background:The randomized phase 3 TIVO-3 study met the primary endpoint of improved PFS with tivozanib (TIVO) vs sorafenib (SOR) in patients with relapsed/refractory mRCC with fewer dose reductions, interruptions and discontinuations despite a longer time on therapy. Greater insight into temporal characteristics of treatment-emergent adverse events (TEAEs) may enable proactive supportive care strategies and improve patient experience.
Methods:Updated safety from the previously reported TIVO-3 study with a data cutoff August 15, 2019, was analyzed by treatment arm for time-to-onset (TTO, days [d]) of the most commonly reported TEAEs, and TTO of first dose reduction, interruption, and discontinuation occurring with TIVO and SOR. Duration of TEAE (median d and IQ range), and rate of dose reduction, interruption, or discontinuation due to the TEAE was calculated for each arm.
Results: Patients in the safety analysis randomly assigned to TIVO (n = 173) or SOR (n = 170) received 11.9 and 6.7 cycles, or 336 and 192 mean days of treatment exposure, respectively. Incidence of any Gr, Gr >3, and TTO of any Gr TEAE of special interest occurring with >20% frequency in either arm is shown in Table 1. While TIVO was associated with less Gr>3 diarrhea, rash and PPE and more HTN than SOR, there were few differences in the TTO or duration of these TEAEs. Overall, dose reductions, interruptions, and discontinuations due to TEAEs were less frequent with TIVO than SOR, and TTO of first dose reduction (85 vs 45 d), interruption (81 vs 50 d), and discontinuation (114 vs 49 d) was longer for TIVO than SOR. Among those experiencing the same TEAE in either arm, resulting dose modifications were less frequent with TIVO than SOR.
Conclusions: TIVO-3 demonstrated improved PFS with TIVO compared to SOR in mRCC, with longer duration of TIVO exposure, but fewer all Gr and Gr >3 TEAEs. Temporal characteristics of TEAEs were similar, but time to dose modifications was longer with TIVO than SOR. Among those with the same TEAEs, unmodified treatment was continued more often with TIVO than SOR.

Research Funding: AVEO Oncology
Clinical trial information: NCT02627963.


This material on this page is ©2021 American Society of Clinical Oncology, all rights reserved. For more information, please contact licensing@asco.org.



